- Department of Neurosurgery, Shunan Memorial Hospital, Kudamatsu, Yamaguchi, Japan.
DOI:10.25259/SNI_97_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Masaru Honda, Hajime Maeda. Intraoperative hematoma volume can predict chronic subdural hematoma recurrence. 25-May-2021;12:232
How to cite this URL: Masaru Honda, Hajime Maeda. Intraoperative hematoma volume can predict chronic subdural hematoma recurrence. 25-May-2021;12:232. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=10830
Abstract
Background: We routinely measured the exact chronic subdural hematoma (CSDH) volume during single burr hole surgery. To date, several risk factors have been reported for CSDH recurrence, including sex, hematoma volume and degree of midline shift calculated from computed tomography, use of anticoagulants or antiplatelet medications, and alcohol consumption habits. The aim of this study was to clarify whether hematoma volume, in conjunction with other factors, can predict recurrence.
Methods: We retrospectively reviewed the clinical data of 194 consecutive patients with CSDH who underwent single burr hole surgery. The risk factors for recurrence were analyzed based on patients’ sex, age, bilaterality, existence of apparent trauma history, exact intraoperative hematoma volume, and various clinical factors, including preoperative anticoagulant/antiplatelet intake.
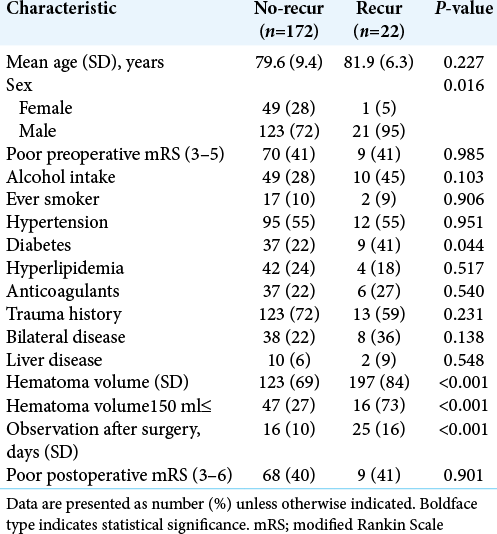
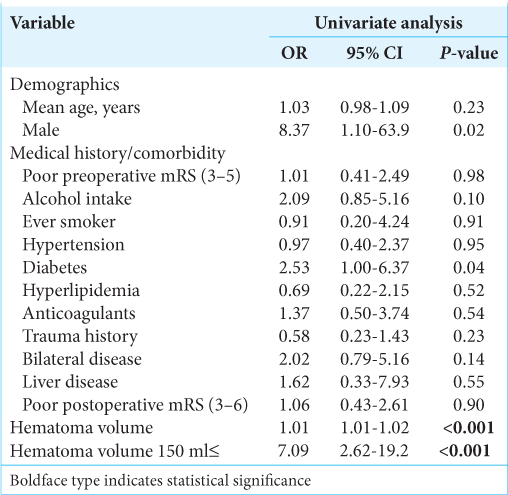
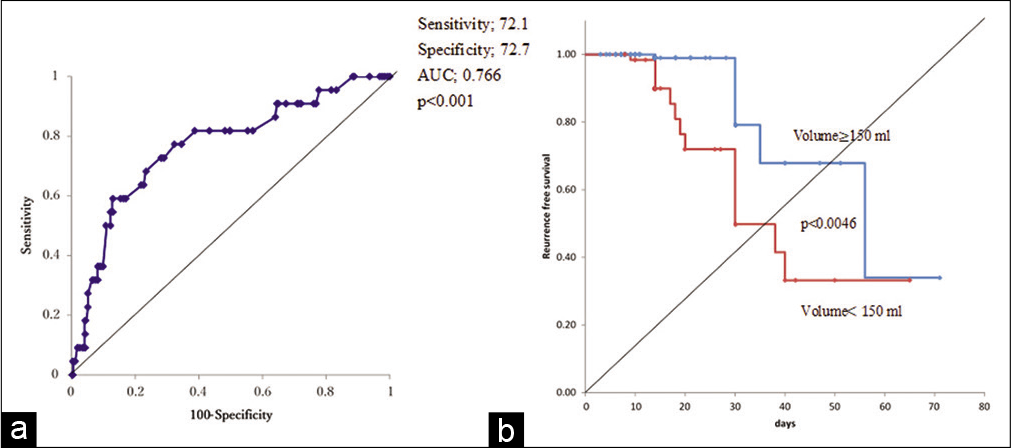
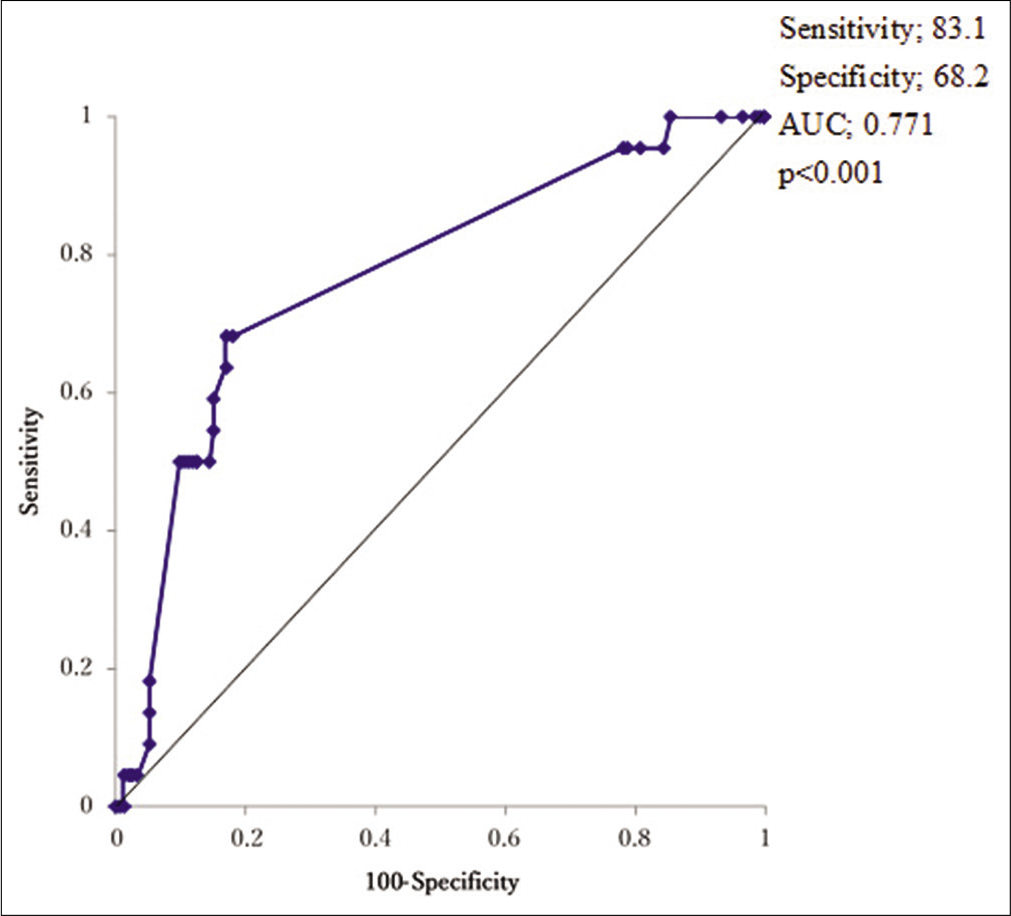
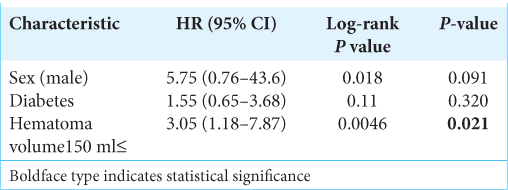
Results: Recurrence occurred in 22 patients (11.3%). Multivariate logistic regression analysis revealed that intraoperative hematoma volume was an independent risk factor for CSDH recurrence (odds ratio [OR], 1.01; 95% confidence interval [CI], 1.01–1.02, P P = 0.049) and diabetes mellitus (DM) (OR: 3.97, 95% CI, 1.34–11.7, P = 0.013). Based on receiver operating characteristics analysis, the cutoff value of the hematoma volume predicting CSDH recurrence was 150 ml (sensitivity and specificity of 72.7% and 72.1%, respectively; area under the curve: 0.7664, 95% CI: 0.654–0.879, P P P = 0.0046, P = 0.021). Follow-up periods after surgery were significantly longer for cases with recurrence than for non-recurrence cases (24.8 ± 11.5 vs. 15.9 ± 9.7 days), and the recurrence prediction cutoff value was 17 days, with a sensitivity and specificity of 83.1% and 68.2%, respectively (AUC: 0.7707, 95% CI: 0.6695–0.8720, P
Conclusion: Intraoperative hematoma volume could be a predictive value for CSDH recurrence.
Keywords: Chronic subdural hematoma, Hematoma volume, Recurrence, Risk factors
INTRODUCTION
Chronic subdural hematoma (CSDH) is one of the most frequent diseases encountered by neurosurgeons.[
The aims of this study were to identify preoperative predictive factors for recurrence, and we hypothesized that intraoperative hematoma volume was also associated with CSDH recurrence and could be included as a risk factor. If possible, we also studied the exact postsurgical drainage times and volumes of patients, to clarify the appropriate drainage duration.
This study was conducted in accordance with the principles outlined in the Declaration of Helsinki and was approved by the Shunan Memorial Hospital Institutional Review Board. The review board approved that the need for informed consent was waived, given the retrospective nature of the study.
MATERIALS AND METHODS
Surgical techniques
All patients underwent single burr-hole surgery, and closed system drainage was performed under local anesthesia. The hematoma was evacuated as follows. Just after cutting the outer membrane of the hematoma, the stand-by aspirator vacuumed the hematoma as much as possible. When voluntary hematoma flow was confirmed to have stopped, the aspirated hematoma volume was measured using a commercial surgical bucket. A drainage tube was then inserted posteroinferiorly into the hematoma cavity. At the extent of insertion, manual aspiration was performed gently using an injection tube. Aspirated hematoma volume was calculated as the total volume of the hematoma. In cases of bilateral disease, the hematoma volume was calculated by combining both sides. Irrigation was seldom performed.[
Subjects and methods
We retrospectively analyzed the clinical records of patients who were treated over a period of 8 years (January 1, 2013, to December 31, 2020). Age, sex, and medical history, including medication use, alcohol intake, and smoking habits, were recorded. Recurrence was defined as the reappearance of neurological signs and/or symptoms with CT evidence of increased hematoma cavity volume, which was re-treated with burr hole surgery and was counted as a case. Postoperative follow-up termination was based on radiological and clinical findings.
Statistical analysis
Data are presented as mean ± standard deviation for continuous variables and number (percentage) for categorical variables. Univariate analysis was performed using the Mann–Whitney U-test, while either Pearson’s Chi-square test or Fisher’s exact test was used to examine the presence of an association between the study variables and CSDH recurrence. The Mann–Whitney U-test was used for non-categorical variables (i.e., age, hematoma volume, drainage volume, and hours). Factors associated with recurrence in the univariate analysis (P < 0.20) were entered into a multivariate logistic regression analysis. Two-tailed P < 0.05 was considered statistically significant. Odds ratios (ORs) were estimated for magnitude of effect with 95% confidence intervals (CIs). The Kaplan–Meier survival curve was plotted, and the log-rank test was conducted to compare the time to recurrence. Statistical analyses were performed using BellCurve for Excel (Social Survey Research Information Co., Ltd., Tokyo, Japan).
RESULTS
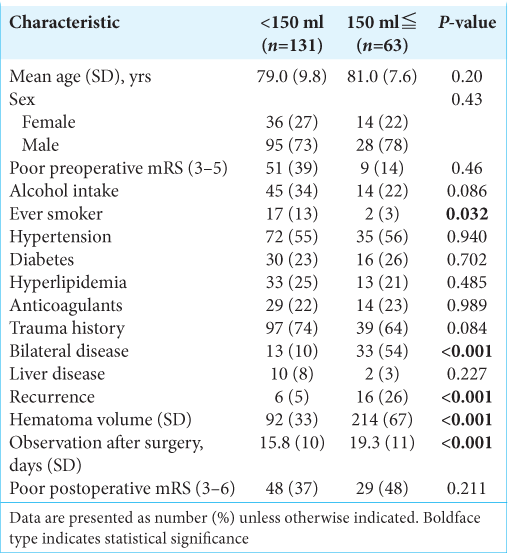
The results are summarized in [
Figure 1:
Hematoma volume and recurrence. (a) Receiver operating characteristic curve analysis for recurrence after burr hole surgery of chronic subdural hematoma reveals cut-off value of intraoperative hematoma volume. (b) Kaplan–Meier curves of recurrence-free survival after burr hole surgery for intraoperative hematoma volume >150 ml shows higher recurrence rate.
[
Recurrence cases had longer observation periods than non-recurrence cases (24.8 ± 11.5 vs. 15.9 ± 9.7 d, respectively, P < 0.001), and ROC analysis revealed that the recurrence predictive cutoff value of follow-up periods was 17 days, with a sensitivity and specificity of 83.1% and 68.2%, respectively [AUC: 0.7707, 95% CI: 0.6695–0.8720, P < 0.001,
DISCUSSION
The annual incidence of CSDH is 3.4–20.6/100,000, but this increases to 7.35–127.1/100,000 in the elderly group (over 65 years or 80 years of age).[
The predictive factors for recurrence were categorized as (1) Clinically: male sex, age, alcohol intake, coagulation abnormality (including anticoagulant/antiplatelet intake, liver diseases, and malignancy), DM; (2) Radiologically: hyper density hematoma on CT, bilateral disease, brain atrophy, midline shift, hematoma thickness, large hematoma volume, air, and/or residual hematoma volume after surgery; (3) symptomatically: aphasia, long symptom duration, and residual headache after surgery; and (4) methodologically: no drainage and irrigation or non-irrigation.[
Most previous studies have estimated hematoma volume by CT and/or MRI.[
To simplify the calculation of hematoma volume, we counted cases on an individual basis, unified the volume of both sides in bilateral disease and omitted complicated counts on the case and sides. Most volumetric studies using CT/ MRI are one-dimensional (width) or two-dimensional studies, while three-dimensional analysis before surgery is a time-consuming method from the perspective of emergency medicine. Further development of computational analysis will solve these issues, but still be a virtual reality method.[
CSDH is an apparently male-dominant disease, which was reflected in the results of this study; several hypotheses for this have been proposed, including that males are more likely to experience trauma and complications and a theoretical estrogen protective effect on microcapillary development on the hematoma membrane in females.[
We reached the consensus that DM is a strong predictive factor for CSDH recurrence, as previously reported.[
Bilateral disease and alcohol consumption habits were associated with recurrence, although without statistical significance, but the former would physically increase hematoma volume with poor brain re-expansion compared to unilateral disease, and the latter leads to liver dysfunction and results in a poor anticoagulation status in the human body, which may explain this recurrence tendency.[
Whether AC/AT is a risk factor for CSDH recurrence is controversial, as is the matter of discontinuation and resumption, or constant administration of these drugs during CSDH surgery.[
There is no consensus yet on an optimal follow-up imaging protocol, although several efforts have been made to ensure the surveillance of recurrence.[
The use of saline irrigation of the hematoma cavity is dependent on the surgeon’s preference, and whether irrigation is a risk factor for recurrence is also controversial.[
CSDH will become the most common neurosurgical disease in 10 years, and long-term outcomes of elderly patients with CSDH are poorer than expected, and we therefore do not recognize this disease as a benign disease in the senile developing world. CSDH is an important cause of morbidity and mortality and is associated with reduced long-term survival.[
Most reports regarding CSDH surgery seldom mentioned the actual drainage time and volume, only mentioning whether it was “overnight,” “1-day,” “2-days,” or “48-h.”[
Atorvastatin, steroids, the ACE inhibitor, tranexamic acid, or Chinese herb Gorei-san use has been recommended to reduce recurrence.[
As a result, we recommend the following methods for CSDH surgery: (1) single burr-hole surgery, (2) under local anesthesia, (3) recording intraoperative hematoma volume, (4) around 20 h drainage without irrigation (5) early discharge followed by CT examination after 1 week, and (6) with protective Gorei-san subscription.
This study has several limitations. The first was the lack of precise CT assessments of recurrence before and after surgery. Second, this study only followed a relatively small number of patients. Third, this was a retrospective study conducted at a single center. Fourth, all patients underwent the same surgery and anesthesia protocol (single burr hole under local anesthesia); thus, there was no comparison with other methods. Finally, the follow-up CT scans were evaluated according to predetermined protocols, and the termination of follow-up was based on the physician’s decision.
CONCLUSION
This study showed that intraoperative hematoma volume measurement could be a predictive value for CSDH recurrence. The patients followed up for more than 17 days and with hematoma volume > 150 mL should be carefully observed for recurrence, in addition to male patients and DM patients. Further prospective multicenter studies are required to evaluate these findings.
Author contributions to the study and manuscript preparation include the following: conception and design: Honda. Acquisition of data: Honda. Analysis and interpretation of data: Honda. Drafting the article: Honda Critically revising the article: All authors Reviewed submitted version of the manuscript: All authors. Statistical analysis: Honda.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Abouzari M, Rashidi A, Rezaii J, Esfandiari K, Asadollahi M, Aleali H. The role of postoperative patient posture in the recurrence of traumatic chronic subdural hematoma after burr-hole surgery. Neurosurgery. 2007. 61: 794-7
2. Adachi A, Higuchi Y, Fujikawa A, Machida T, Sueyoshi S, Harigaya K. Risk factors in chronic subdural hematoma: Comprehension of irrigation with artificial cerebrospinal fluid and normal saline in a cohort analysis. PLoS One. 2014. 9: e103703
3. Altaf I, Shams S, Vohra AH. Radiological predictors of recurrence of chronic subdural hematoma. Pak J Med Sci. 2018. 34: 194-7
4. Ban SP, Hwang G, Byoun HS, Kim T, Lee SU, Bang JS. Middle meningeal artery embolization for chronic subdural hematoma. Radiology. 2018. 286: 992-9
5. Basler D, Farooq S, Mehmood T, Reyes M, Samadani U. Actual and projected incidence rates for chronic subdural hematomas in United States Veterans administration and civilian populations. J Neurosurg. 2015. 123: 1209-15
6. Chen FM, Wang K, Xu KL, Wang L, Zhan TX, Cheng F. Predictors of acute intracranial hemorrhage and recurrence of chronic subdural hematoma following burr hole drainage. BMC Neurol. 2020. 20: 92
7. Hammer A, Tregubow A, Kerry G, Schrey M, Hammer C, Steiner HH. Predictors for recurrence of chronic subdural hematoma. Turk Neurosurg. 2017. 27: 756-62
8. Hashimoto T, Ohashi T, Watanabe D, Koyama S, Namatame H, Izawa H. Usefulness of embolization of the middle meningeal artery for refractory chronic subdural hematomas. Surg Neurol Int. 2014. 4: 104
9. Honda M, Maeda H. Chronic subdural hematoma; necessity of irrigation and preventive factors of recurrence. Int J Curr Med Pharm Res. 2017. 3: 2389-91
10. Huang YH. Volume of chronic subdural haematoma: Is it one of the radiographic factors related to recurrence?. Injury. 2014. 45: 1327-31
11. Ivamoto H, Lemos H, Atallah A. Surgical treatment for Chronic subdural hematomas: A comprehensive systemic review. World Neurosurg. 2016. 86: 399-418
12. Jang KM, Choi HH, Mun HY, Nam TK, Park YS, Kwon JT. Critical depressed brain volume influences the recurrence of chronic subdural hematoma after surgical evacuation. Sci Rep. 2010. 10: 1145
13. Javadi SA, Naderi F, Javadi AM. The optimal surgical approach for treatment of chronic subdural hematoma: Questionnaire assessment of practice in Iran and review of the literature. Acta Med Iran. 2015. 53: 617-21
14. Kim J, Moon J, Kim T, Ahn S, Hwang G, Bang J. Risk factor analysis for the recurrence of chronic subdural hematoma: A review of 368 consecutive surgical cases. Korean J Neurotrauma. 2015. 11: 63-9
15. Kim SU, Lee DH, Kim YI, Yang SH, Sung JH, Cho CB. Predictive factors for recurrence after burr-hole craniostomy of chronic subdural hematoma. J Korean Neurosurg Soc. 2017. 60: 701-9
16. Kudo H, Kuwamura K, Izawa I, Sawa H, Tamaki N. Chronic subdural hematoma in elderly people: Present status on Awaji Island and epidemiological prospect. Neurol Med Chir (Tokyo). 1992. 32: 207-9
17. Lee KS. How to treat chronic subdural hematoma? Past and now. J Korean Neurosurg Soc. 2019. 62: 144-52
18. Liu W, Bakker NA, Groen JM. Chronic subdural hematoma: A systemic review and meta-analysis of surgical procedures. J Neurosurg. 2014. 121: 665-73
19. Neto JF, Araujo JLV, Ferraz VR, Haddad L, Veiga JC. Chronic subdural hematoma: Epidemiological and prognostic analysis of 176 cases. Rev Col Bras Cir. 2015. 42: 283-7
20. Okada Y, Akai T, Okamoto K, Iida T, Takata H, Iizuka H. A comparative study of the treatment of chronic subdural hematoma--burr hole drainage versus burr hole irrigation. Surg Neurol. 2002. 57: 405-9
21. Qiu S, Zhuo W, Sun C, Su Z, Yan A, Shen L. Effects of atorvastatin on chronic subdural hematoma: A systematic review. Medicine. 2017. 96: e7290
22. Ro HW, Park SK, Jang DK, Yoon WS, Jang KS, Han YM. Preoperative predictive factors for surgical and functional outcomes in chronic subdural hematoma. Acta Neurochir (Wien). 2016. 158: 135-9
23. Rovlias A, Theodoropoulos S, Papoutsakis D. Chronic subdural hematoma: surgical management and outcome in 986 cases: A classification and regression tree approach. Surg Nerol Int. 2015. 6: 127
24. Santarius T, Kirkpatrick PJ, Ganesan D, Chia HL, Jalloh I, Smielewski P. Use of drains versus no drains after burr-hole evacuation of chronic subdural haematoma: A randomised controlled trial. Lancet. 2009. 374: 1067-73
25. Schwarz F, Loos F, Dünisch P, Sakr Y, Safatli DA, Kalff R. Risk factors for reoperation after initial burr hole trephination in chronic subdural hematomas. Clin Neurol Neurosurg. 2015. 138: 66-71
26. Stanišić M, Hald J, Rasmussen IA, Pripp AH, Ivanović J, Kolstad F. Volume and densities of chronic subdural haematoma obtained from CT imaging as predictors of postoperative recurrence: A prospective study of 107 operated patients. Acta Neurochir (Wien). 2013. 155: 323-33
27. Stavrinou P, Katsigiannis S, Lee JH, Hamisch C, Krischek B, Mpotsaris A. Risk factors for chronic subdural hematoma recurrence identified using quantitative computed tomography analysis of hematoma volume and density. World Neurosurg. 2017. 99: 465-70
28. Takahashi S, Yamauchi T, Yamamura T, Ogishima T, Arai T. Proposal of treatment strategies for bilateral chronic subdural hematoma based on laterality of treated hematoma. Asian J Neurosurg. 2018. 13: 1134-9
29. Torihashi K, Sadamasa N, Yoshida K, Narumi O, Chin M, Yamagata S. Independent predictors for recurrence of chronic subdural hematoma: A review of 343 consecutive surgical cases. Neurosurgery. 2008. 63: 1125-9
30. Uda H, Nagm A, Ichinose T, Onishi Y, Yoshimura M, Tsuruno T. Burr hole drainage without irrigation for chronic subdural hematoma. Surg Neurol Int. 2020. 11: 89
31. Uno M, Toi H, Hirai S. Chronic subdural hematoma in elderly patients: Is this disease benign?. Neurol Med Chir (Tokyo). 2017. 57: 402-9
32. Weigel R, Schmiedek P, Krauss JK. Outcome of contemporary surgery for chronic subdural haematoma: Evidence-based review. J Neurol Neurosurg Psychiatry. 2003. 74: 937-43
33. Xu C, Chen S, Yuan L, Jing Y. Burr-hole irrigation with closed-system drainage for the treatment of chronic subdural hematoma: A meta-analysis. Neurol Med Chir (Tokyo). 2016. 56: 62-8
34. Yamamoto H, Hirashima Y, Hamada H, Hayashi N, Origasa H, Endo S. Independent predictors of recurrence of chronic subdural hematoma: Results of multivariate analysis performed using a logistic regression model. J Neurosurg. 2003. 98: 1217-21