- Department of Surgery, Division of Neurosurgery, Faculty of Medicine, King Abdulaziz University Hospital, Jeddah, Saudi Arabia
- Department of Spine Surgery, King Abdulaziz Medical City, Ministry of National Guard, Jeddah, Saudi Arabia
Correspondence Address:
Soha A. Alomar, Department of Surgery, Division of Neurosurgery, Faculty of Medicine, King Abdulaziz University Hospital, Jeddah, Saudi Arabia.
DOI:10.25259/SNI_179_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Faisal A. Sukkar1, Sultan F. Albalawi2, Tala S. AlSindi1, Soha A. Alomar1. Intraoperative iatrogenic seizure induced by transcranial motor-evoked potential during spinal surgery: A case report and review of the literature. 01-Nov-2024;15:391
How to cite this URL: Faisal A. Sukkar1, Sultan F. Albalawi2, Tala S. AlSindi1, Soha A. Alomar1. Intraoperative iatrogenic seizure induced by transcranial motor-evoked potential during spinal surgery: A case report and review of the literature. 01-Nov-2024;15:391. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13201
Abstract
Background: Intraoperative neuromonitoring is an essential tool for detecting early intraoperative neurological changes during spinal surgery. Only rarely do seizures occur during transcranial motor-evoked potentials (TcMEP).
Case Description: A 44-year-old male presented with a magnetic resonance (MR)-documented L5-S1 T2-hyperintense intradural mass that heterogeneously enhanced with Gadolinium and extended through the right S1 neural foramen. Utilizing transcranial motor-evoked potential (Tc-MEP) before the skin incision, the patient developed the 1st seizure that lasted for 2 min. The 2nd seizure occurred after the initial incision and lasted for around 15 min; at this point, the procedure was terminated. After brain MR studies documented no structural lesion and other etiologies of seizures were ruled out, the patient underwent an uneventful resection of the L5–S1 spinal lesion.
Conclusion: Although the risk of seizures from Tc-MEP is very low, it is crucial to be aware of this potential side effect. If they occur, surgical procedures should be aborted and diagnostic studies performed to rule out the presence of structural lesions and/or other reasons for seizure activity.
Keywords: Complication, Epilepsy, Motor-evoked potential, Neuromonitoring, Seizure
INTRODUCTION
Monitoring motor-evoked potentials (MEPs) and performing transcranial electrical stimulation during spinal surgery can decrease the risk of intraoperative and, therefore, postoperative new motor deficits.[
CASE REPORT
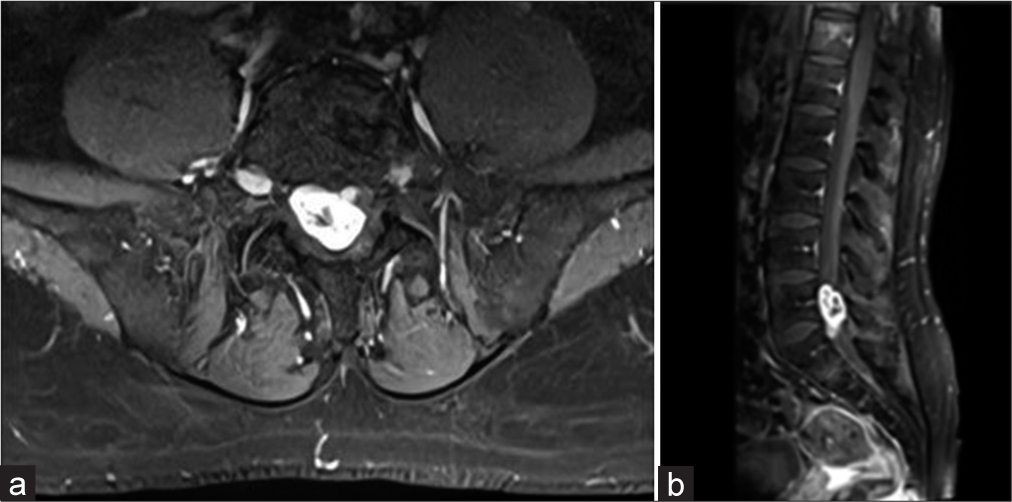
A 44-year-old male with 6 months of back pain had a lumbar computed tomography (CT) that showed an isodense intradural right-sided mass at the L5–S1 level. The magnetic resonance (MR) demonstrated that it was hyperintense on the T2-weighted image, heterogeneously enhanced with contrast, and extended through the right S1 foramen [
However, before the skin incision, the patient developed focal seizures involving the left upper and lower extremities that lasted for 2 min; they were terminated by anesthesia’s increasing the dose of propofol. After exposing the spinous process of L5, a second focal left-sided seizure occurred that lasted 15 min; it was treated with an increased dose of propofol. Midazolam was added, and the operation was aborted. The urgent brain CT and MR studies were both negative for a structural lesion, and laboratory studies were normal. The patient was kept intubated overnight on continuous electroencephalogram (EEG) monitoring; he had no recurrent seizures, and he was given and maintained on levetiracetam 500 mg twice daily. Two days later, he underwent an uneventful Tc-MEP monitored L5-S1 laminectomy for resection of a Schwannoma; he was discharged 2 days later on routine antiepileptic prophylaxis. Six months later, the patient still has not exhibited any additional seizure activity.
DISCUSSION
Rare seizures with Tc-MEP monitoring during spine surgery
Tc-MEP (i.e., involving short-term, high-voltage electrical stimulation) for monitoring spinal surgery rarely results in seizure activity. However, Tc-MEP may contribute to other more common adverse such as cardiac arrhythmias, movement-related injuries, tongue lacerations, scalp burns, and headaches.[
We found one case for Sokhal et al. in our literature review, which involved a 35-year-old man who had T4-T5 laminectimy for an intradural extramedullary tumor with Tc-MEP. The man experienced a 30-second seizure involving all of his limbs; the seizure was stopped by stopping the Tc-MEP and giving the patient 2 mg of IV midazolam, which prevented the seizure but allowed the surgery to proceed.[
Factors predisposing to seizure activity when utilizing TcMEP monitoring for spine surgery
Many factors may increase the risk of seizures occurring during Tc-MEP monitoring of spinal surgery. These include a history of epilepsy, hypoxia, hypercarbia, hypoglycemia, and electrolyte imbalance. The following anesthesia medications can also lower seizure thresholds: nitrous oxide, enflurane, etomidate, ketamine, propofol, morphine, meperidine, fentanyl, sufentanil, alfentanil, and local anesthesia.[
Tc-MEP most likely etiology of patient’s intraoperative seizures
Transcranial MEP is most likely the etiology of seizures that we observed during the first surgery; however, both the brain CT and MR studies were negative, and the postoperative EEG monitoring showed no additional seizure activity. Further, the EEG 3 months after the patient’s discharge showed no interictal epileptiform activity. We, therefore, concluded that the two intraoperative seizures were triggered by the Tc-MEP used to monitor this patient’s spinal surgery.
CONCLUSION
Although the risk of seizures from Tc-MEP is very low, it is crucial to be aware of this risk if repetitive abnormal movements/seizures occur during spinal surgery.
Ethical approval
The Institutional Review Board has waived the ethical approval for this study.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Burbridge MA, Nguyen V, Gi Min J, Jaffe RA, Ahuja B, Shah AD. Intraoperative transcranial motor-evoked potential stimulation does not seem to cause seizures. J Neurosurg Anesthesiol. 2021. 33: 351-5
2. Deletis V, Sala F. Intraoperative neurophysiological monitoring of the spinal cord during spinal cord and spine surgery: A review focus on the corticospinal tracts. Clin Neurophysiol. 2008. 119: 248-64
3. Kobylarz EJ. Monitoring of electroencephalography during transcranial electrical motor evoked potentials. Epilepsia. 2005. 46: 309-10
4. MacDonald DB. Safety of intraoperative transcranial electrical stimulation motor evoked potential monitoring. J Clin Neurophysiol. 2002. 19: 416-29
5. Modica PA, Tempelhoff R, White PF. Pro-and anticonvulsant effects of anesthetics (Part II). Anesth Analg. 1990. 70: 433-44
6. Neuloh G, Pechstein U, Cedzich C, Schramm J. Motor evoked potential monitoring with supratentorial surgery. Neurosurgery. 2004. 54: 1061-72
7. Sala F, Lanteri P. Brain surgery in motor areas: The invaluable assistance of intraoperative neurophysiological monitoring. J Neurosurg Sci. 2003. 47: 79-88
8. Sokhal S, Goyal K, Sokhal N, Kumar N, Kedia S. Iatrogenic seizures during intraoperative transcranial motor-evoked potential monitoring. Asian J Neurosurg. 2019. 14: 967-9
9. Ulkatan S, Jaramillo AM, Téllez MJ, Kim J, Deletis V, Seidel K. Incidence of intraoperative seizures during motor evoked potential monitoring in a large cohort of patients undergoing different surgical procedures. J Neurosurg. 2017. 126: 1296-302