- Division of Neurosurgery, Foothills Medical Centre, University of Calgary, Calgary, Alberta, Canada
Correspondence Address:
Alim P. Mitha
Division of Neurosurgery, Foothills Medical Centre, University of Calgary, Calgary, Alberta, Canada
DOI:10.4103/2152-7806.173567
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Avery M, Chehab S, Wong JH, Mitha AP. Intraoperative indocyanine green videoangiography to guide decision making regarding need for vessel bypass: A case report and technical note. Surg Neurol Int 07-Jan-2016;7:
How to cite this URL: Avery M, Chehab S, Wong JH, Mitha AP. Intraoperative indocyanine green videoangiography to guide decision making regarding need for vessel bypass: A case report and technical note. Surg Neurol Int 07-Jan-2016;7:. Available from: http://surgicalneurologyint.com/surgicalint_articles/intraoperative-indocyanine-green-videoangiography-to-guide-decision-making-regarding-need-for-vessel-bypass-a-case-report-and-technical-note/
Abstract
Background:Indocyanine green (ICG) videoangiography is an intraoperative technique recently used in vascular neurosurgery to assess the presence or absence of blood flow during critical times of a procedure. These include, but are not limited to, detecting whether daughter branches or perforators are patent after placing a vascular clip or determining whether an aneurysm has been completely isolated from the cerebral circulation after clipping. We present a case of a less-commonly reported application of ICG videoangiography involving the selection of a vessel to act as the bypass recipient once the need is identified during the surgical treatment of a complex intracranial aneurysm.
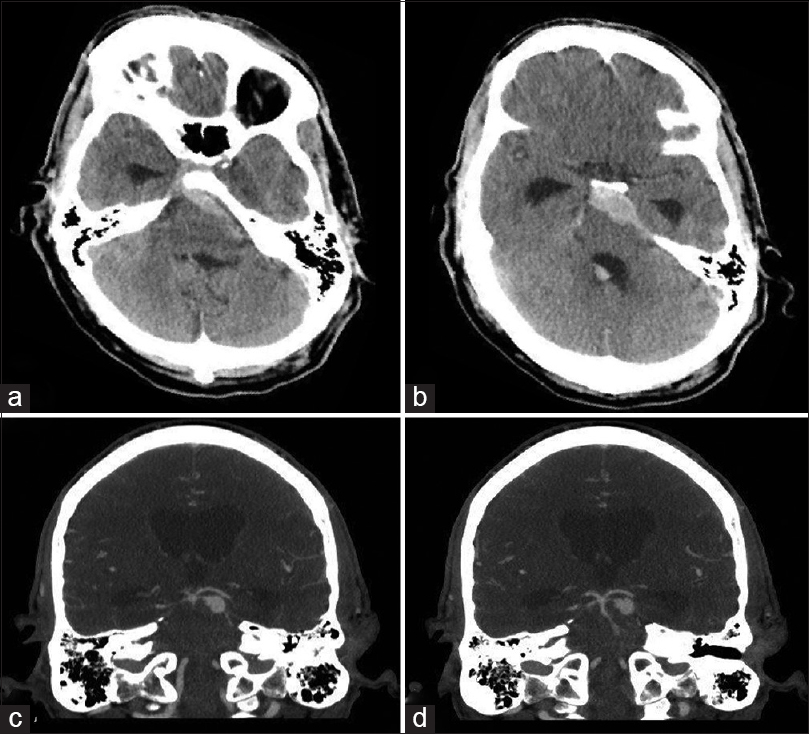
Case Description:A 51-year-old male presented with a ruptured dissecting superior cerebellar artery (SCA) aneurysm that had two branches arising from the dome. Due to the difficult morphology of this aneurysm, a superficial temporal artery to SCA bypass was planned. We used ICG videoangiography to identify the branch that had insufficient retrograde flow via collateral circulation, to which the bypass was performed, followed by the isolation of the aneurysm from the cerebral circulation using permanent surgical clips.
Conclusion:Our case represents a possible use of ICG videoangiography during the operative treatment of a difficult aneurysm. Our patient suffered no infarcts postoperatively. In the correct clinical context, this method represents a possible treatment option for complex aneurysms requiring a bypass.
Keywords: Dissecting intracranial aneurysm, extracranial-intracranial bypass, indocyanine green videoangiography, revascularization
BACKGROUND AND IMPORTANCE
Intraoperative angiography is a useful tool in cerebrovascular neurosurgery. One commonly used method is indocyanine green (ICG) videoangiography. ICG is a cyanine dye that has a peak spectral absorption of between 780 and 805 nm, with an emission spectrum peaking between 820 and 835 nm.[
CLINICAL PRESENTATION
History
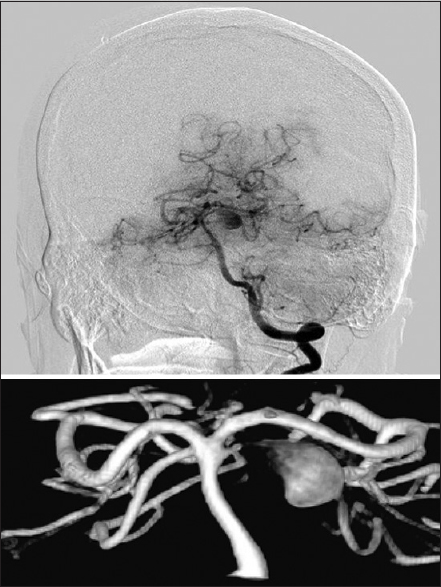
A healthy 51-year-old male presented to our institution with a headache, complete left oculomotor nerve palsy and increasing lethargy secondary to a ruptured 10 mm left proximal superior cerebellar artery (SCA) dissecting aneurysm with the two branching vessels emerging from its distal aspect [
Operation
On post-SAH day 10, the patient was taken to the operating room for definitive management of the aneurysm. A left-sided orbitozygomatic craniotomy was performed in the usual fashion with care to expose and isolate 12 cm of the superficial temporal artery (STA), which was identified transcutaneously with a handheld Doppler. The STA was protected with a papaverine-soaked gauze. Using a subfrontal approach and with microscopic magnification, the left opticocarotid complex was identified. The left posterior communicating artery was then followed to the ipsilateral posterior cerebral artery. The proximal SCA was then identified and dissected for later clip placement.
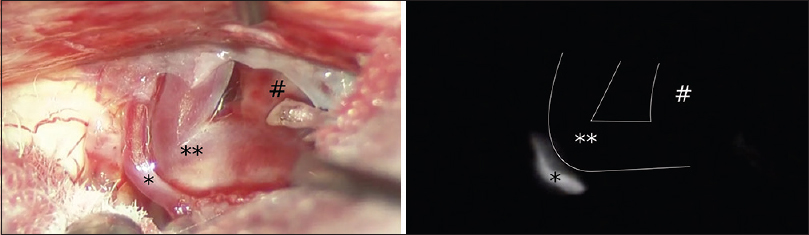
We then followed a left subtemporal corridor to identify the anuerysm and two branches of the SCA emerging from its distal aspect. The trochlear nerve was divided to improve exposure; since the patient had presented with complete oculomotor nerve palsy with associated ptosis, it was felt that dividing the fourth nerve would result in no significant additional deficit. As expected, two branches of the SCA were found to be arising from the aneurysm, and temporary clips were placed proximally on each branch. Using intraoperative ICG videoangiography, we found that the smaller of the two SCA branches had retrograde flow while the larger did not, indicating a lack of collateral flow into the larger SCA branch [
Figure 3
Intraoperative microscopic view of aneurysm complex viewed during left subtemporal approach (right = anterior, up = inferior) (above). Identical intraoperative view during indocyanine green videoangeography after applying temporary clips proximally on the branches of the superior cerebellar artery (below). Smaller branch (*) is seen fluorescing, while larger branch (**) and aneurysm dome (#) are not seen. The location of these nonfluorescing structures is outlined in white
To perform the bypass, a temporary clip was placed on the proximal aspect of the exposed left STA, and the vessel was longitudinally transected distally. After clearing the vessel of its adventitia, the STA was laid down along the floor of the middle cranial fossa to the aneurysm site. Temporary clips were positioned on the proximal and distal aspects of the larger SCA branch. The bypass was performed end-to-side on the SCA branch between the clips with 10-0 nylon sutures. The three temporary clips were removed, establishing flow through the bypass. Finally, permanent clips were applied to the SCA proximal to the aneurysm, as well as on the proximal aspects of the two SCA branches as they emerged from the aneurysm, thus isolating the aneurysm from the cerebral circulation. The bone was replaced, and the craniotomy was closed in the usual fashion.
Postoperative course
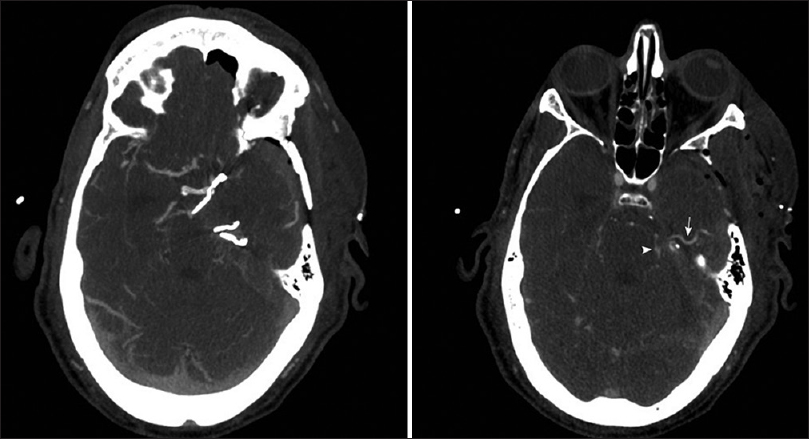
A postoperative CTA demonstrated patency of the bypass and no residual filling of the excluded dissecting aneurysm [
Figure 4
Postoperative axial computed tomography angiogram demonstrating complete clip occlusion of the left superior cerebellar artery aneurysm. A single clip was placed proximal to the aneurysm and one clip was placed proximally on each of the distal branches (left). The superficial temporal artery (arrow) is patent and feeding into the patent larger superior cerebellar artery (arrowhead) branch (right). No cerebellar infarcts were noted on postoperative imaging
DISCUSSION
Treatment options for complex aneurysms include open surgical and endovascular approaches. Open surgical approaches include clipping, proximal vessel occlusion, trapping of the aneurysm with or without distal bypass, or wrapping. Endovascular options include coiling with or without stent or balloon assistance, flow diversion or parent artery occlusion. In our particular case of a distal dissecting SCA aneurysm, clipping was not an option as the aneurysm involved both branches of the SCA, and so clip occlusion would have likely resulted in an SCA territory infarct. Furthermore, endovascular treatment was not a great option as coil embolization of a dissecting aneurysm is technically very challenging. Using a flow-diverting stent would require prior knowledge of collateral circulation to the efferent branches, as only one branch could be stented.
Cerebral revascularization is an important option in the surgical treatment of these complex aneurysms. One of the main issues with the procedure, however, is patient selection. This procedure carries with it greater technical difficulty and additional risks. Accurately identifying patients and situations where a bypass will be of benefit, and perhaps more importantly, when it is not warranted, will undoubtedly improve patient outcomes.[
There have been several reports of the intraoperative use of ICG videoangiography during aneurysm surgery. In many cases, this technique has successfully identified the patency of a completed bypass or perforator vessels.[
Despite the success, in this case, there are several limitations to the use of intraoperative ICG. For example, the available line of sight can restrict the complete visualization of an aneurysm and branch complex and may be affected by calcification or thrombosis.[
CONCLUSION
Cerebral revascularization via direct bypass is a useful treatment option for some complex aneurysms for which conventional approaches pose exceptionally high risks. It is well known that intraoperative ICG videoangiography can provide the surgeon valuable information regarding bypass patency. Less-commonly reported is the ability to use this technique in prebypass assessments to evaluate candidate recipient vessels for bypass based on either collateral flow or identification of the distal branches of interest.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Alander JT, Kaartinen I, Laakso A, Pätilä T, Spillmann T, Tuchin VV. A review of indocyanine green fluorescent imaging in surgery. Int J Biomed Imaging 2012. 2012. p.
2. Chaloupka JC, Putman CM, Awad IA. Endovascular therapeutic approach to peripheral aneurysms of the superior cerebellar artery. AJNR Am J Neuroradiol. 1996. 17: 1338-42
3. Esposito G, Durand A, van Doormaal T, Regli L. Selective-targeted extra-intracranial bypass surgery in complex middle cerebral artery aneurysms: Correctly identifying the recipient artery using indocyanine green videoangiography. Neurosurgery. 2012. 71: ons274-85
4. Gruber A, Dorfer C, Bavinzski G, Standhardt H, Ferraz-Leite H, Knosp E. Superselective indocyanine green angiography for selective revascularization in the management of peripheral cerebral aneurysms. AJNR Am J Neuroradiol. 2012. 33: E36-7
5. Kalani MY, Rangel-Castilla L, Ramey W, Nakaji P, Albuquerque FC, McDougall CG. Indications and results of direct cerebral revascularization in the modern era. World Neurosurg. 2015. 83: 345-50
6. Kim DL, Cohen-Gadol AA. Indocyanine-green videoangiogram to assess collateral circulation before arterial sacrifice for management of complex vascular and neoplastic lesions: Technical note. World Neurosurg. 2013. 79: 404.e1-6
7. Lane B, Bohnstedt BN, Cohen-Gadol AA. A prospective comparative study of microscope-integrated intraoperative fluorescein and indocyanine videoangiography for clip ligation of complex cerebral aneurysms. J Neurosurg. 2015. 122: 618-26
8. Linskey ME, Jungreis CA, Yonas H, Hirsch WL, Sekhar LN, Horton JA. Stroke risk after abrupt internal carotid artery sacrifice: Accuracy of preoperative assessment with balloon test occlusion and stable xenon-enhanced CT. AJNR Am J Neuroradiol. 1994. 15: 829-43
9. Raabe A, Nakaji P, Beck J, Kim LJ, Hsu FP, Kamerman JD. Prospective evaluation of surgical microscope-integrated intraoperative near-infrared indocyanine green videoangiography during aneurysm surgery. J Neurosurg. 2005. 103: 982-9
10. Towle EL, Richards LM, Kazmi SM, Fox DJ, Dunn AK. Comparison of indocyanine green angiography and laser speckle contrast imaging for the assessment of vasculature perfusion. Neurosurgery. 2012. 71: 1023-30
11. Woitzik J, Horn P, Vajkoczy P, Schmiedek P. Intraoperative control of extracranial-intracranial bypass patency by near-infrared indocyanine green videoangiography. J Neurosurg. 2005. 102: 692-8