- Department of Neurosurgery, Sapporo Medical University, Sapporo, Japan
- Department of Neurosurgery, Maronie Street Neurological Clinic, Sapporo, Japan
- Division of Clinical Engineering, Sapporo Medical University, Sapporo, Japan.
Correspondence Address:
Rei Enatsu, Department of Neurosurgery, Sapporo Medical University, Sapporo, Japan.
DOI:10.25259/SNI_303_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Chie Kamada1, Rei Enatsu1, Aya Kanno1, Satoko Ochi2, Shoto Yamada3, Ryota Sato1, Ryohei Chiba1, Nobuhiro Mikuni1. Intraoperative nerve stimulation during vagal nerve stimulator placement. 01-Sep-2023;14:312
How to cite this URL: Chie Kamada1, Rei Enatsu1, Aya Kanno1, Satoko Ochi2, Shoto Yamada3, Ryota Sato1, Ryohei Chiba1, Nobuhiro Mikuni1. Intraoperative nerve stimulation during vagal nerve stimulator placement. 01-Sep-2023;14:312. Available from: https://surgicalneurologyint.com/surgicalint-articles/12519/
Abstract
Background: Vagal nerve stimulation (VNS) is a palliative treatment for refractory epilepsy and intraoperative nerve stimulation is applied to the vagal and other nerves to prevent electrode misplacement. We evaluated these thresholds to establish intraoperative monitoring procedures for VNS surgery.
Methods: Forty-six patients who underwent intraoperative nerve stimulation during VNS placement were enrolled. The vagal nerve and other exposed nerves were electrically stimulated during surgery, and muscle contraction was confirmed by electromyography of the vocal cords and visual recognition of cervical muscle contraction. The nerve thresholds and the most sensitive parts of the vagal nerve were analyzed retrospectively.
Results: The stimulation of vagal nerves induced vocal cord responses in all 46 patients; the median thresholds of the most sensitive parts and all parts were 0.2 mA (range: 0.05–0.75 mA) and 0.25 mA (range: 0.15–1.5 mA), respectively. The medial middle region was identified as the most sensitive part of the vagal nerve in the majority of participants (82.5%). In 11 patients, other cervical nerves were stimulated and sternohyoid muscle contraction was induced with a median threshold of 0.35 mA (range: 0.1–0.7 mA) in eight patients, while sternocleidomastoid muscle contraction was induced with a median threshold of 0.2 mA (range: 0.1–0.2 mA) in three.
Conclusion: Intraoperative stimulation of vagal nerves induces vocal cord responses with locational variations, and the middle part stimulation could minimize the stimulus intensities. The nerves innervating the sternohyoid and sternocleidomastoid muscles may be exposed during the procedure. Knowledge of these characteristics will enhance the effectiveness of this technique in future applications.
Keywords: Epilepsy, Intraoperative stimulation, Sternocleidomastoid muscle, Sternohyoid muscle, Vagal nerve stimulation
INTRODUCTION
Vagal nerve stimulation (VNS) is a palliative treatment for refractory epilepsy, which aims to reduce the frequency and severity of seizures. This therapy is indicated for patients with resection-unsuitable epilepsy, or as an additional treatment option.[
We have previously reported an intraoperative monitoring technique to prevent electrode misplacement.[
MATERIALS AND METHODS
Patients
We enrolled 46 patients who underwent intraoperative nerve stimulation during VNS placement between March 2013 and May 2022 at our institute. The patients’ ages ranged from 5 to 68 years and 24 were male. This study was approved by the Institutional Review Board of Sapporo Medical University Graduate School of Medicine (IRB# 292–101). Our monitoring protocol was previously introduced using a part of the current data.[
Intraoperative nerve stimulation
We performed vocal cord monitoring to map the vagal nerve following a previously described protocol.[
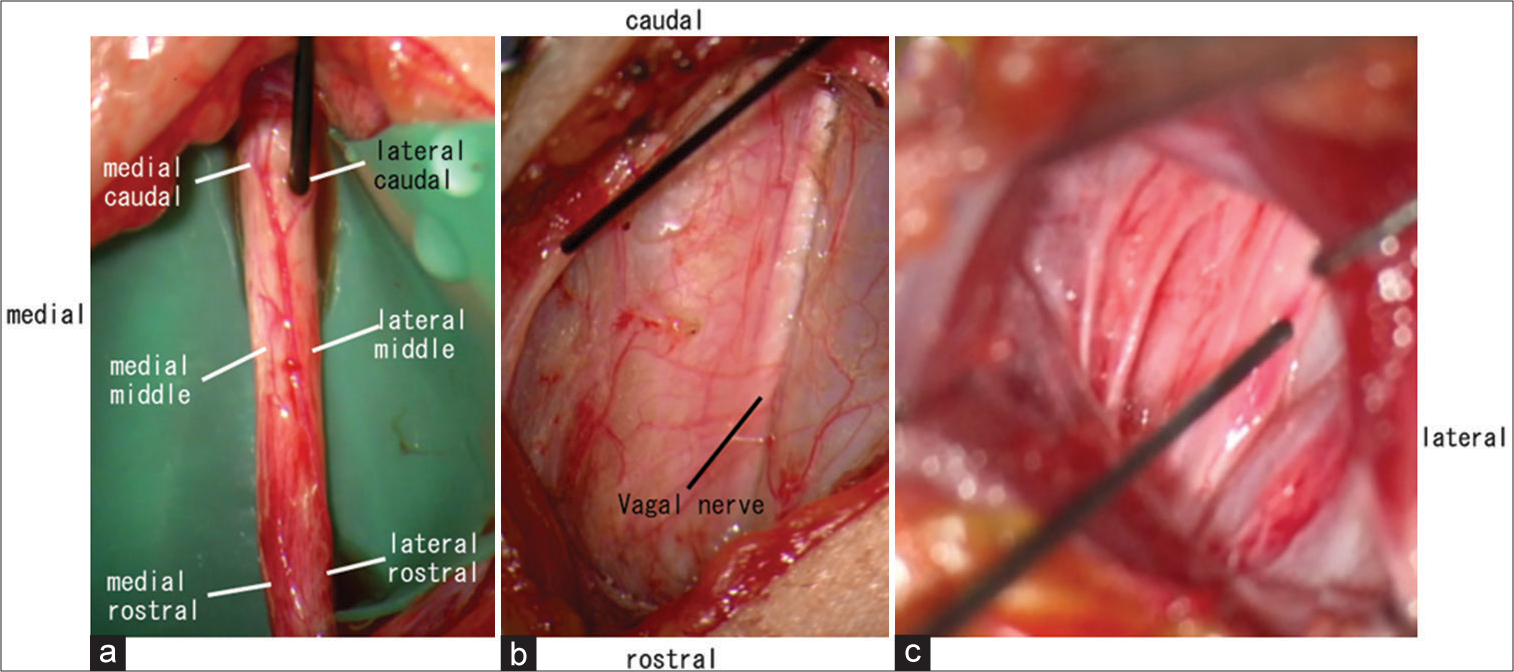
Figure 1:
(a) Six points on the exposed vagal nerve (medial caudal, lateral caudal, medial middle, lateral middle, medial rostral, and lateral rostral) were stimulated, and the electromyographic activity of the vocal cords was recorded to check the thresholds. (b) The exposed nerve was stimulated and sternohyoid muscle contraction was induced. (c) The exposed nerve was stimulated and sternocleidomastoid muscle contraction was induced.
Data analysis
Among the six points on the vagal nerve (medial caudal, lateral caudal, medial middle, lateral middle, medial rostral, and lateral rostral), the point at which the lowest intensity stimulus-induced vocalis muscle activity was regarded as the most sensitive. The most sensitive point was located and referred to in the operative record or intraoperative monitoring reports of 40 patients. The thresholds of the most sensitive parts and all parts were evaluated. Furthermore, the other exposed nerves were stimulated during surgery, and the corresponding thresholds were evaluated.
RESULTS
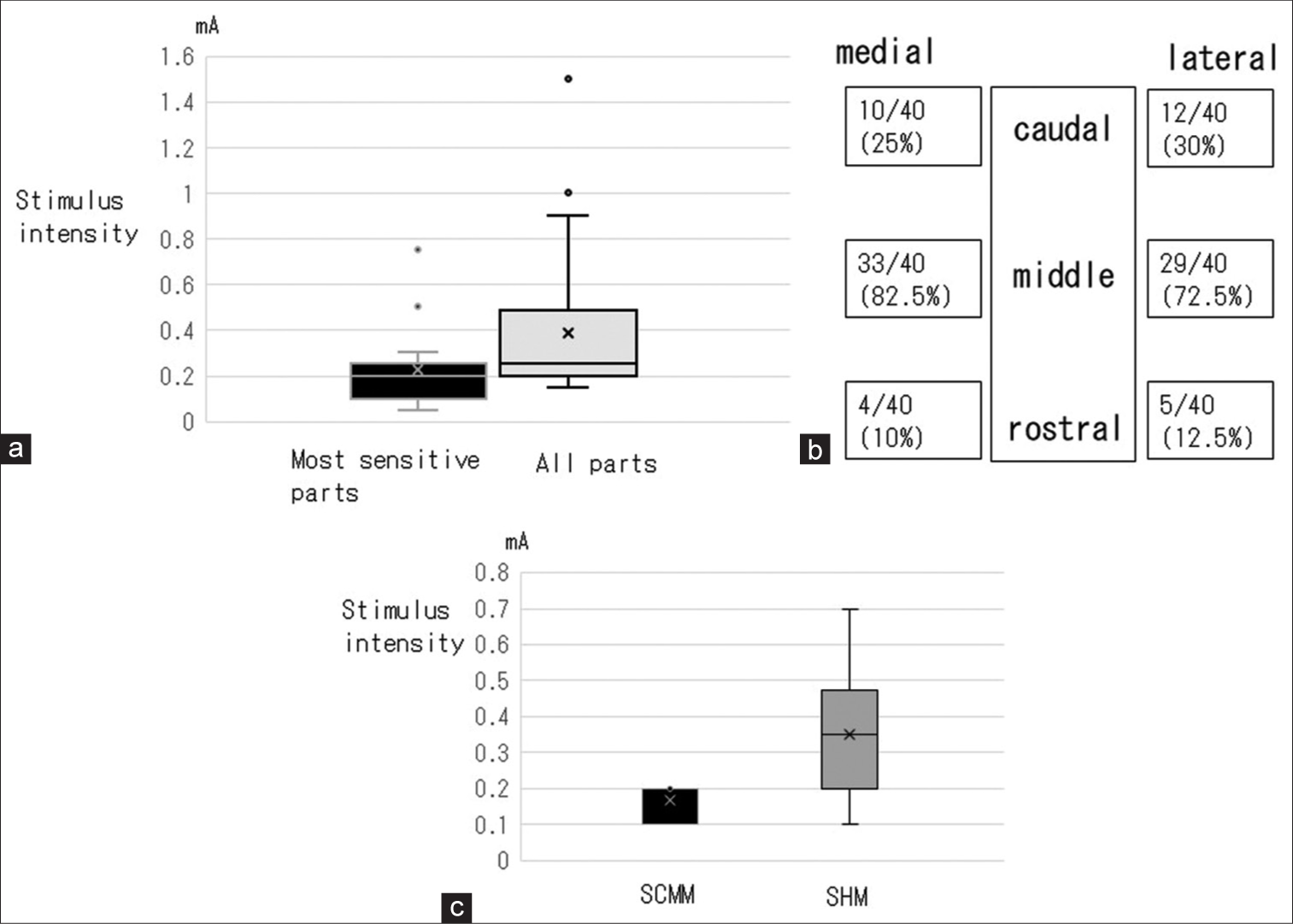
VNS induced vocal cord responses in all 46 patients. None of the patients experienced complications suggestive of electrode misplacement, such as contraction of the neck muscles. The median thresholds of the most sensitive parts and all parts were 0.2 mA (range: 0.05–0.75 mA) and 0.25 mA (range: 0.15–1.5 mA), respectively [
Figure 2:
(a) The threshold of the most sensitive parts and all parts of the exposed vagal nerve to induce a vocal cord response. (b) The number and percentage of times each part was identified as the most sensitive part in all 40 patients. The medial middle parts were the most sensitive part of the vagal nerve in the majority of participants, followed by the lateral middle, lateral caudal, medial caudal, lateral rostral, and medial rostral parts. (c) The threshold of nerves to induce sternocleidomastoid and sternohyoid muscle contraction. SCMM: Sternocleidomastoid muscle, SHM: Sternohyoid muscle.
The other nerves were exposed and stimulated. Contraction of the sternohyoid muscle was induced in eight patients at a median threshold of 0.35 mA (range: 0.1–0.7 mA) [
DISCUSSION
In the present study, VNS induced vocal cord responses in all 46 patients, and the locational variation of threshold ranged between 0.05 mA and 1.5 mA. The middle parts were most sensitive in 72.5–82.5 % of patients, the caudal parts in 25–30%, and the rostral parts in 10–12.5%. These results indicate that the middle part stimulation could minimize the stimulus intensities to induce vocal code responses. The vocal cord is innervated by the vagal nerve through the superior and recurrent laryngeal nerves. The recurrent laryngeal nerve branches directly originate from the vagal nerve, and the superior laryngeal nerve originates from the inferior ganglion.[
Other nerves surrounding the vagal nerve were also exposed and stimulated in this procedure. Sternohyoid muscle contraction was induced in eight patients and sternocleidomastoid muscle contraction in three patients. The sternohyoid muscle is innervated by the ansa cervicalis,[
This study had several limitations. The first was the small number of stimulated nerves innervating the sternohyoid and sternocleidomastoid muscles; unfortunately, our results were insufficient to establish a threshold for these nerves. Second, the course of these nerves varies widely, and precise anatomical identification is difficult. Third, the vagal nerve might have been rotated by dissection. Therefore, the six points of VNS may have shifted from the original anatomical position. We laid the rubber sheet under the vagus nerve before the stimulation. The placement of a rubber sheet could align the vagus nerve and prevent nerve rotation. Fourth, visual inspection has the limitation of identifying all contracted muscles, which might have caused some muscle responses to be overlooked.
CONCLUSION
This study revealed that intraoperative VNS successfully induced vocal cord responses with a locational variation of the threshold between 0.05 mA and 1.5 mA. The middle part of the exposed vagal nerve had the highest probability of inducing a vocal cord response. Furthermore, the nerves innervating the sternohyoid and sternocleidomastoid muscles, such as the ansa cervicalis, cervical plexus, and accessory nerve, can be exposed and differentiated from the vagal nerves by electrical stimulation of 0.1–0.7 mA. A deeper knowledge of these characteristics will enhance the effectiveness and reliability of this technique.
Declaration of patient consent
Patients’ consent not required as patients’ identities were not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The author(s) confirms that there was no use of Artificial Intelligence (AI)-Assisted Technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Brennan PA, Alam P, Ammar M, Tsiroyannis C, Zagkou E, Standring S. Sternocleidomastoid innervation from an aberrant nerve arising from the hypoglossal nerve: A prospective study of 160 neck dissections. Surg Radiol Anat. 2017. 39: 205-9
2. Chiba R, Enatsu R, Ochi S, Yamada S, Sasagawa A, Suzuki H. Intraoperative monitoring for vagus nerve stimulation. World Neurosurg. 2019. 131: 191-3
3. Giordano F, Zicca A, Barba C, Guerrini R, Genitori L. Vagus nerve stimulation: Surgical technique of implantation and revision and related morbidity. Epilepsia. 2017. 58: 85-90
4. Gopalakrishnan CV, Kestle JR, Connolly MB The. “vagal ansa”: A source of complication in vagus nerve stimulation. J Neurosurg Pediatr. 2015. 15: 535-8
5. Iriarte J, Artieda J, Alegre M, Schlumberger E, Urrestarazu E, Pastor MA. Spasm of the sternocleidomastoid muscle induced by vagal nerve stimulation. Neurology. 2001. 57: 2319-20
6. Kahlow H, Olivecrona M. Complications of vagal nerve stimulation for drug-resistant epilepsy: A single center longitudinal study of 143 patients. Seizure. 2013. 22: 827-33
7. Kruse E, Olthoff A, Schiel R. Functional anatomy of the recurrent and superior laryngeal nerve. Langenbecks Arch Surg. 2006. 391: 4-8
8. Labuschagne J, Mutyaba D, Nel J, Casieri C. Intra-operative monitoring as an adjuvant to standard vagus nerve stimulation implantation. Childs Nerv Syst. 2021. 37: 3809-16
9. Meguid EA, Agawany AE. An anatomical study of the arterial and nerve supply of the infrahyoid muscles. Folia Morphol (Warsz). 2009. 68: 233-43
10. Mikuni N, Satow T, Taki J, Nishida N, Enatsu R, Hashimoto N. Endotracheal tube electrodes to map and monitor activities of the vagus nerve intraoperatively. Technical note. J Neurosurg. 2004. 101: 536-40
11. Mikuni N, Usui N, Otsubo H, Kawai K, Kishima H, Maehara T. Current status and future objectives of surgical therapies for epilepsy in Japan. Neurol Med Chir (Tokyo). 2021. 61: 619-28
12. Prades JM, Gavid M, Dubois MD, Dumollard JM, Timoshenko AT, Peoc’h M. Surgical anatomy of the ansa cervicalis nerve: Which branch to use for laryngeal reinnervation in humans?. Surg Radiol Anat. 2015. 37: 139-45
13. Rychlicki F, Zamponi N, Cesaroni E, Corpaci L, Trignani R, Ducati A. Complications of vagal nerve stimulation for epilepsy in children. Neurosurg Rev. 2006. 29: 103-7
14. Shaffer MJ, Jackson CE, Szabo CA, Simpson CB. Vagal nerve stimulation: Clinical and electrophysiological effects on vocal fold function. Ann Otol Rhinol Laryngol. 2005. 114: 7-14
15. Spuck S, Tronnier V, Orosz I, Schönweiler R, Sepehrnia A, Nowak G. Operative and technical complications of vagus nerve stimulator implantation. Neurosurgery. 2010. 67: 489-94
16. Usami K, Kawai K, Sonoo M, Saito N. Scalp-recorded evoked potentials as a marker for afferent nerve impulse in clinical vagus nerve stimulation. Brain Stimul. 2013. 6: 615-23
17. Vaughn BV, Bernard E, Lannon S, Mann B, D’Cruz OF, Shockley W. Intraoperative methods for confirmation of correct placement of the vagus nerve stimulator. Epileptic Disord. 2001. 3: 75-8
18. Yokoyama R, Akiyama Y, Enatsu R, Suzuki H, Suzuki Y, Kanno A. The immediate effects of vagus nerve stimulation in intractable epilepsy: An intra-operative electrocorticographic analysis. Neurol Med Chir (Tokyo). 2020. 60: 244-51