- Department of Neurosurgery, Aga Khan University Hospital, Karachi, Sindh, Pakistan,
- Department of Neurosurgery, Southmead Hospital, North Bristol, United Kingdom,
- Department of Radiology, National Medical Centre, Karachi, Sindh, Pakistan,
- Department of Radiology, Aga Khan University Hospital, Karachi, Sindh, Pakistan,
- Department of Radiology, Hyperfine, Guilford, Connecticut, United States.
Correspondence Address:
Syed Ather Enam, Department of Neurosurgery, Aga Khan University Hospital, Karachi, Sindh, Pakistan.
DOI:10.25259/SNI_124_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ahmed Altaf1, Muhammad Waqas Saeed Baqai2, Faiza Urooj1, Muhammad Sami Alam3, Hafiza Fatima Aziz1, Fatima Mubarak4, Edmond Knopp5, Khan Siddiqui5, Syed Ather Enam1. Intraoperative use of ultra-low-field, portable magnetic resonance imaging – first report. 23-Jun-2023;14:212
How to cite this URL: Ahmed Altaf1, Muhammad Waqas Saeed Baqai2, Faiza Urooj1, Muhammad Sami Alam3, Hafiza Fatima Aziz1, Fatima Mubarak4, Edmond Knopp5, Khan Siddiqui5, Syed Ather Enam1. Intraoperative use of ultra-low-field, portable magnetic resonance imaging – first report. 23-Jun-2023;14:212. Available from: https://surgicalneurologyint.com/surgicalint-articles/12380/
Abstract
Background: Intraoperative use of portable magnetic resonance imaging (pMRI) has become a valuable tool in a surgeon’s arsenal since its inception. It allows intraoperative localization of tumor extent and identification of residual disease, hence maximizing tumor resection. Its utility has been widespread in high-income countries for the past 20 years, but in lower-middle-income countries (LMIC), it is still not widely available due to several reasons, including cost constraints. The use of intraoperative pMRI may be a cost-effective and efficient substitute for conventional MRI machines. The authors present a case where a pMRI device was used intraoperatively in an LMIC setting.
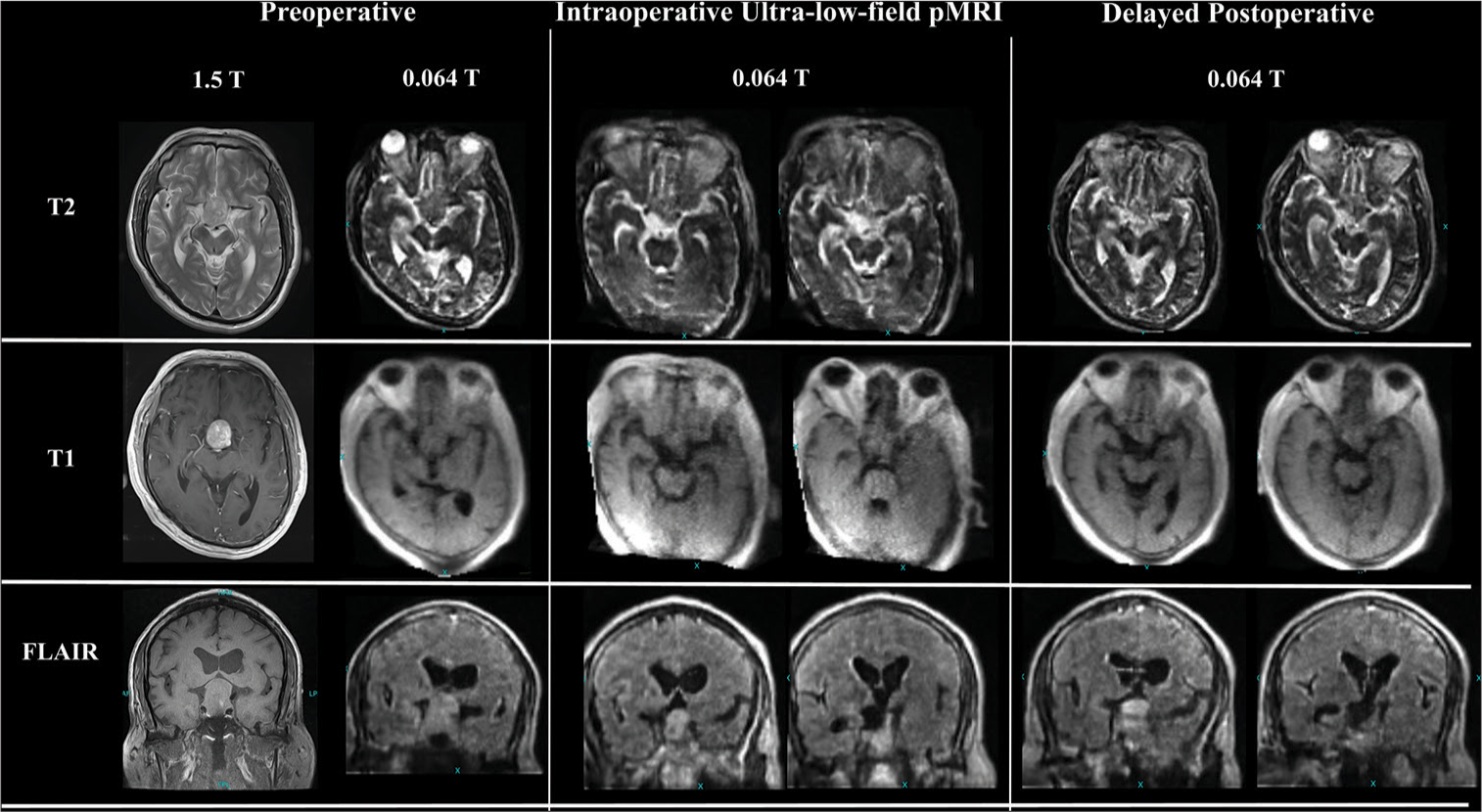
Case Description: The authors performed a microscopic transsphenoidal resection of a sellar lesion with intraoperative imaging using the pMRI system on a 45-year-old man with a nonfunctioning pituitary macroadenoma. Without the need for an MRI suite or other MRI-compatible equipment, the scan was conducted within the confinements of a standard operating room. Low-field MRI showed some residual disease and postsurgical changes, comparable to postoperative high-field MRI.
Conclusion: To the best of our knowledge, our report provides the first documented successful intraoperative transsphenoidal resection of a pituitary adenoma using an ultra-low-field pMRI device. The device can potentially enhance neurosurgical capacity in resource-constrained settings and improve patient outcomes in developing country.
Keywords: Brain tumors, Portable magnetic resonance imaging (Portable MRI), Intraoperative magnetic resonance imaging (Intraoperative MRI), Ultra-low field magnetic resonance imaging (Ultra-low-field MRI)
INTRODUCTION
Pituitary adenomas account for 10–20% of all intracranial pathologies and the mainstay of treatment is surgery, most commonly through the transsphenoidal approach.[
With recent development in technology and advances in neuroimaging, it has now become possible to conduct MRI intraoperatively. There are several intraoperative adjuncts available that enable surgeons to conduct safer and more effective tumor removal. One of the techniques that have been used in high-income countries (HIC) over the last two decades is incorporating an MRI into the procedure and assessing the extent of tumor resection intraoperatively. However, health-care delivery in a low-resource setting is fundamentally different from the more well-funded Western settings. Given their limited resources, hospitals in lower-middle-income countries (LMIC) are not privileged enough to dedicate an entire MRI suite for operating room (OR) purposes, let alone procure one. In addition to cost constraints, the World Health Organization estimates that 70% of medical equipment coming from developed countries does not work in hospitals in developing countries due to a lack of trained personnel, limitations with infrastructure, and a lack of spare parts or support for equipment.[
The United States Food and Drug Administration (FDA) recently approved the Hyperfine Swoop, an ultra-low-field portable MRI (pMRI), for use in the clinical setting. Its small footprint, low cost, accessibility, intuitiveness, and low magnetic field (0.064 Tesla) can help break barriers toward the provision of top-quality patient care in LMICs for improved health outcomes. We present the first case of pMRI being used intraoperatively for transsphenoidal resection of pituitary macroadenoma in an LMIC setting.
CASE REPORT
A 52-year-old man presented with complaints of headache and blurring of vision in the right eye for the past 1 year, with gradual deterioration of vision. MRI showed typical findings of pituitary macroadenoma [
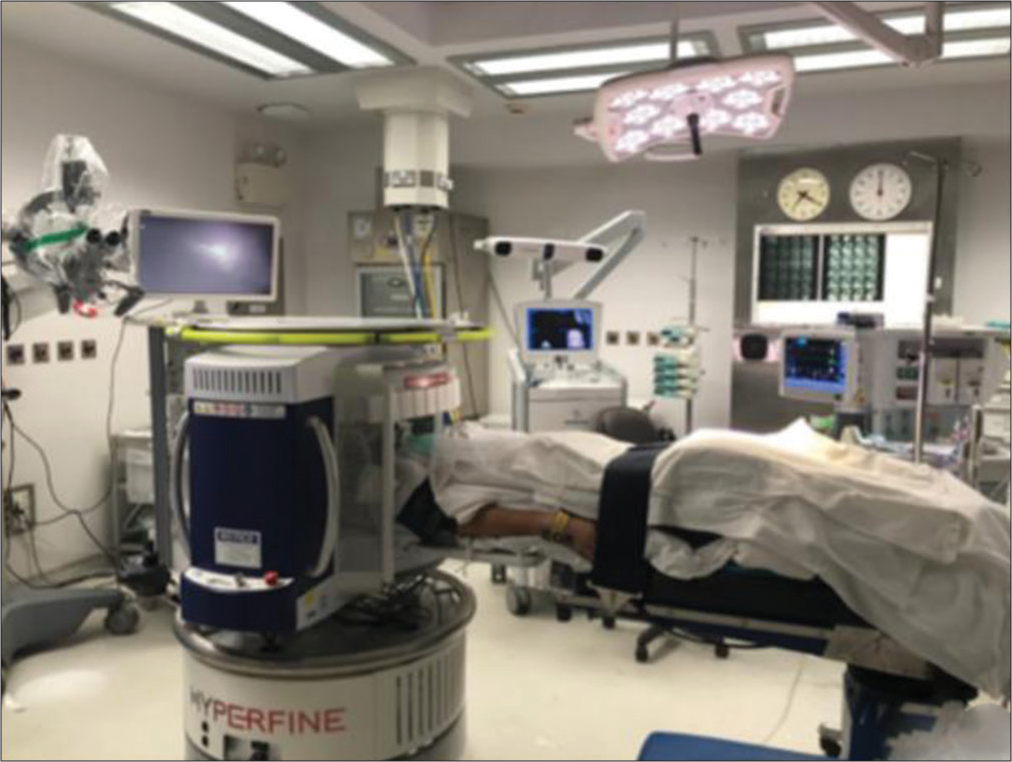
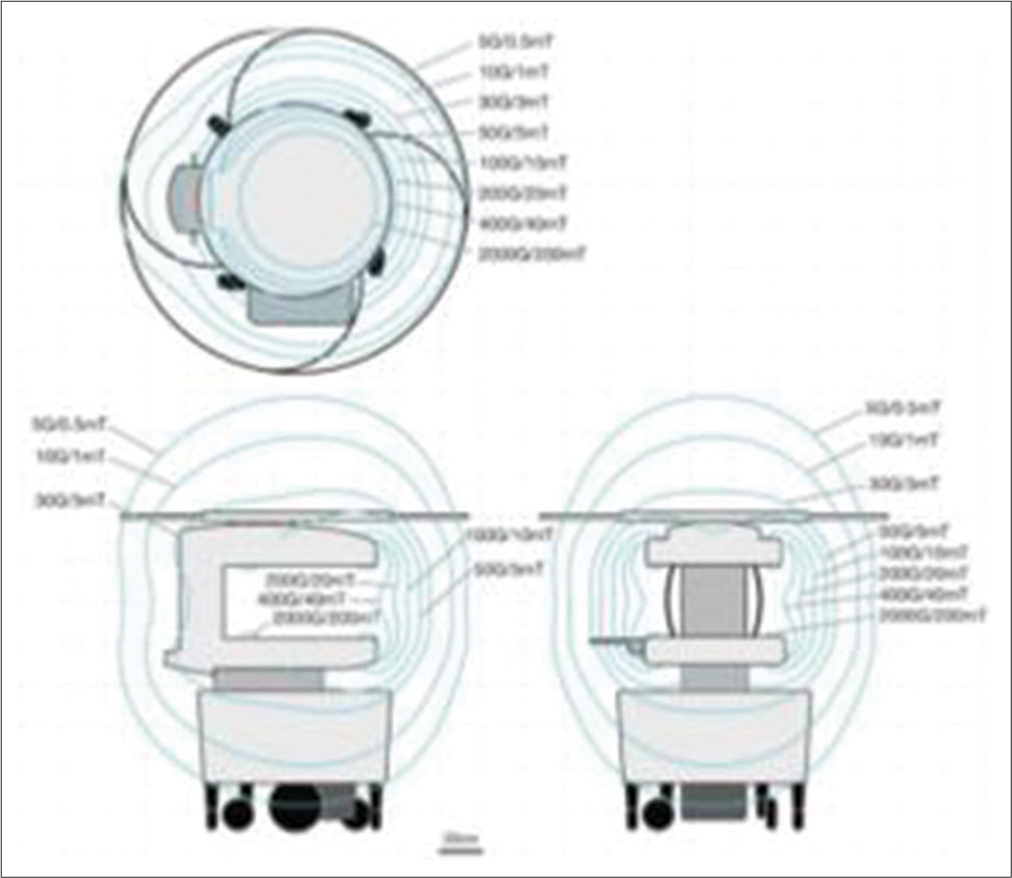
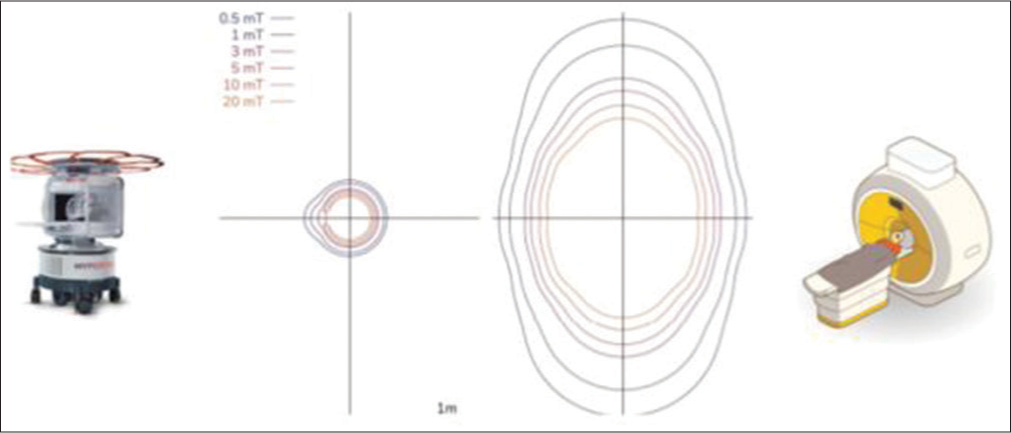
With a small footprint of less than 1.5 m × 1.5 m (5 ft x 5 ft), our pMRI machine requires minimal space and was parked in a corner inside the OR, with its ultra-low-field (0.064 T) magnet enclosed in a secure steel housing. Standard surgical instruments were used during the procedure, which includes microscopes, retractors, and drills. In addition, the monitors and anesthesia machines were not required to be MRI-compatible. After anticipated safe tumor resection, the patient underwent an intra-operative ultra-low-field pMRI scan to determine the extent of any residual tumor. This motor-powered pMRI scanner, controlled by a joystick, enables effortless maneuvering by a single technician, guaranteeing efficient and precise positioning without disrupting the operating room ecosystem. For safety, and to prevent any interference, the 5 Gauss (0.5 mT) field ring was extended. All the OR Equipments, such as IV pumps, ventilators, and other MRI-incompatible devices were placed outside this 5 Gauss (0.5 mT) line [
After the completion of the surgical resection, the patient remained in the same surgical position on a standard operating room table. The pMRI scanner was subsequently brought to the head end of the operating room table, and the technicians carefully positioned the patient’s head inside the machine’s head coil. This coil is specifically designed to enhance the signal-to-noise ratio of the acquired images. The scanner was then connected to a regular 110 V outlet before the initiation of imaging. Our imaging protocol for pituitary brain surgery was composed of a fluid-attenuated inversion recovery coronal, axial T1-weighted image without contrast and T2, Sagittal T2, and diffusion-weighted imaging and apparent diffusion coefficient sequences [
DISCUSSION
Intraoperative MRI (iMRI) is a useful adjuvant technique to maximize the extent of resection because it can provide updated anatomic imaging during surgery to mitigate the changing environment when a brain shift occurs.[
The Swoop pMRI device operates on a magnetic field of 0.064 T. This makes it one of the first devices employing such a low field. This low-field system can be moved into a standard OR and integrated without needing any modification to the surgical environment. The portable nature of this device lowers the possibility of an inadvertent endotracheal tube or central/peripheral line dislodgement brought on by moving patients from the OR to the MRI suite [
However, this portability and small footprint come at the expense of a slightly decreased image quality. Due to its low field, this device has a lower spatial resolution than the conventional 1.5 T device. In our experience and as illustrated in [
A systematic review conducted by Patel et al. revealed that the utilization of low-field-strength (0.15T) pMRI contributes to a significant increase in the rate of gross total resection (GTR), ranging from 3% to 33%, in cases of nonfunctioning pituitary adenomas.[
An increase in OR time is also a factor to consider. The literature states that intraoperative MRI increases OR time by 30–120 min.[
CONCLUSION
This report highlights the importance of the widespread use of pMRI in resource-constricted environments and the technical nuances involved in its implementation. To the best of our knowledge, our report provides the first documented successful transsphenoidal resection of a pituitary adenoma using an ultra-low-field pMRI device intraoperatively. This device will open avenues for further research and its application for other procedures in similar low-income settings. Our experience with this device is intended to reduce barriers to adaptation and allow individuals with less specialized skills to effectively employ these techniques.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
This research was supported by a grant from Hyperfine Research Inc. to the Pakistan Academy of Neurological Surgery (PANS).
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgment
We would like to express our heartfelt gratitude to Dr. Fyezah Jehan for providing invaluable support throughout the project. Additionally, we extend our appreciation to the Department of Pediatrics for their kind provision of facilities and resources, which were instrumental in the successful completion of this project. We would also like to acknowledge the Bill and Melinda Gates Foundation for their generous grant that facilitated the use of the Hyperfine Swoop in our study.
References
1. Berkmann S, Schlaffer S, Nimsky C, Fahlbusch R, Buchfelder M. Follow-up and long-term outcome of nonfunctioning pituitary adenoma operated by transsphenoidal surgery with intraoperative high-field magnetic resonance imaging. Acta Neurochir (Wien). 2014. 156: 2233-43
2. Brochier S, Galland F, Kujas M, Parker F, Gaillard S, Raftopoulos C. Factors predicting relapse of nonfunctioning pituitary macro adenomas after neurosurgery: A study of 142 patients. Eur J Endocrinol. 2010. 163: 193-200
3. Hlavica M, Bellut D, Lemm D, Schmid C, Bernays RL. Impact of ultra-low-field intraoperative magnetic resonance imaging on extent of resection and frequency of tumor recurrence in 104 surgically treated nonfunctioning pituitary adenomas. World Neurosurg. 2013. 79: 99-109
4. Koonjoo N, Zhu B, Bagnall GC, Bhutto D, Rosen MS. Boosting the signal-to-noise of low-field MRI with deep learning image reconstruction. Sci Rep. 2021. 11: 8248
5. Malkin RA. Barriers for medical devices for the developing world. Expert Rev Med Devices. 2007. 4: 759-63
6. Moini J, Avgeropoulos NG, Samsam M, editors. Pituitary tumors. Epidemiology of Brain and Spinal Tumors. United States: Academic Press; 2021. p. 285-322
7. Patel KS, Yao Y, Wang R, Carter BS, Chen CC. Intraoperative magnetic resonance imaging assessment of non-functioning pituitary adenomas during transsphenoidal surgery. Pituitary. 2016. 19: 222-31
8. Schneider JP, Trantakis C, Rubach M, Schulz T, Dietrich J, Winkler D. Intraoperative MRI to guide the resection of primary supratentorial glioblastoma multiforme--a quantitative radiological analysis. Neuroradiology. 2005. 47: 489-500
9. Schulder M, Carmel PW. Intraoperative magnetic resonance imaging: Impact on brain tumor surgery. Cancer Control. 2003. 10: 115-24
10. Sheth KN, Mazurek MH, Yuen MM, Cahn BA, Shah JT, Ward A. Assessment of brain injury using portable, low-field magnetic resonance imaging at the bedside of critically ill patients. JAMA Neurol. 2021. 78: 41-7
11. Sylvester PT, Evans JA, Zipfel GJ, Chole RA, Uppaluri R, Haughey BH. Combined high-field intraoperative magnetic resonance imaging and endoscopy increase extent of resection and progression-free survival for pituitary adenomas. Pituitary. 2015. 18: 72-85
12. Theodosopoulos PV, Leach J, Kerr RG, Zimmer LA, Denny AM, Guthikonda B. Maximizing the extent of tumor resection during transsphenoidal surgery for pituitary macroadenomas: Can endoscopy replace intraoperative magnetic resonance imaging?. J Neurosurg. 2010. 112: 736-43