- Department of Neurosurgery, Faculty of Medicine, University of Tsukuba, Tsukuba, Japan
Correspondence Address:
Eiichi Ishikawa
Department of Neurosurgery, Faculty of Medicine, University of Tsukuba, Tsukuba, Japan
DOI:10.4103/2152-7806.165764
Copyright: © 2015 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ishikawa E, Yamamoto T, Matsuda M, Akutsu H, Zaboronok A, Kohzuki H, Miki S, Takano S, Matsumura A. Intraparenchymal brain lesion biopsy guided by a rigid endoscope and navigation system. Surg Neurol Int 18-Sep-2015;6:149
How to cite this URL: Ishikawa E, Yamamoto T, Matsuda M, Akutsu H, Zaboronok A, Kohzuki H, Miki S, Takano S, Matsumura A. Intraparenchymal brain lesion biopsy guided by a rigid endoscope and navigation system. Surg Neurol Int 18-Sep-2015;6:149. Available from: http://surgicalneurologyint.com/surgicalint_articles/intraparenchymal-brain-lesion-biopsy-guided-by-a-rigid-endoscope/
Abstract
Background:The authors report a continuous case series of navigation-guided rigid endoscopic biopsy via the transcortical route for intraparenchymal brain lesions to assess the feasibility and efficacy of the method.
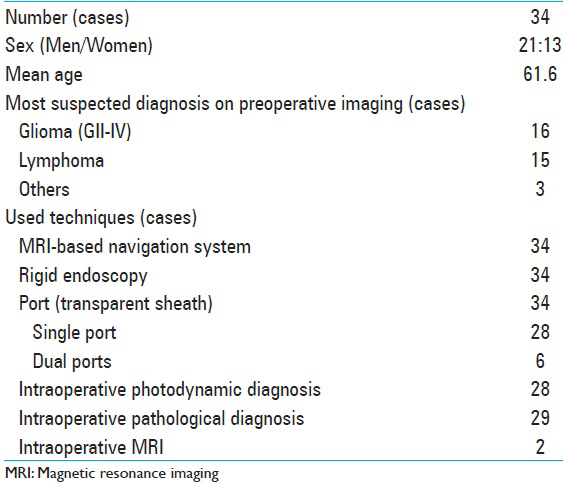
Methods:Thirty-four patients with intraparenchymal brain lesions found on neurovisualization underwent navigation-guided rigid endoscopic biopsy. Most of the preoperative diagnoses were glioma WHO Grade II–IV (16 cases) or malignant lymphoma (15 cases). Intraoperative photodynamic diagnosis and intraoperative pathological diagnosis were used in 28 and 29 cases, respectively. In 2 cases with small and deep lesions, intraoperative magnetic resonance imaging was used for confirming the accuracy of the biopsy point.
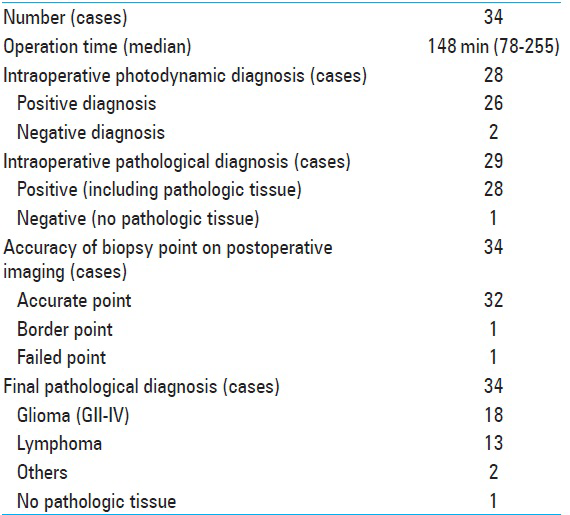
Results:The sampling accuracy determined by postoperative imaging and the definitive diagnosis ratio were 94% (32 out of 34 cases) and 97% (33 out of 34 cases), respectively. There was no postoperative mortality. In 2 patients, mild postoperative permanent morbidity (5.9%), presumably related to this technique, was observed in the early cases in the current group (34 case series).
Conclusion:The method was estimated as safe and feasible for diagnostic tissue sampling of intraparenchymal brain lesions.
Keywords: Endoscopic biopsy, high-grade glioma, malignant lymphoma, needle biopsy
INTRODUCTION
Various methods of intraparenchymal tumor biopsy in the central nervous system have been proposed to date, and can be roughly divided into the following three categories: Needle biopsy, defined as the use of a biopsy needle; endoscopic biopsy, defined as the use of neuroendoscopy; and open biopsy, defined as the use of microscopy through a small craniotomy. These methods differ in details across institutions, including the use of a stereotactic frame, magnetic resonance imaging (MRI)-based navigation system, and rigid fixation. These techniques have advantages and disadvantages related to sampling accuracy, definitive diagnosis ratio, sample volume, and risk of complications.[
Here, we report a continuous case series of navigation-guided rigid endoscopic biopsy via the transcortical route for intraparenchymal brain lesions. The purpose of this retrospective study is to assess the feasibility and efficacy of the rigid endoscopic biopsy.
PATIENTS AND METHODS
In this retrospective study, 34 continuous cases of patients with intraparenchymal brain lesions discovered on MRI who underwent navigation-guided rigid endoscopic biopsy between January 2009 and July 2014 at our hospital were enrolled. The rigid endoscopic biopsy was selected for deep lesions (>3 cm from the brain surface) that were typically noneloquent areas of the brain such as deep frontal tumors. Other biopsy techniques were selected for surface lesions and/or lesions near the major vessels (open biopsy), intra- or para-ventricular lesions (ventriculoscopy or open biopsy), and deep lesions that were located near the eloquent areas (needle biopsy or open biopsy).
The method of navigation-guided rigid endoscopic biopsy was described in previous reports.[
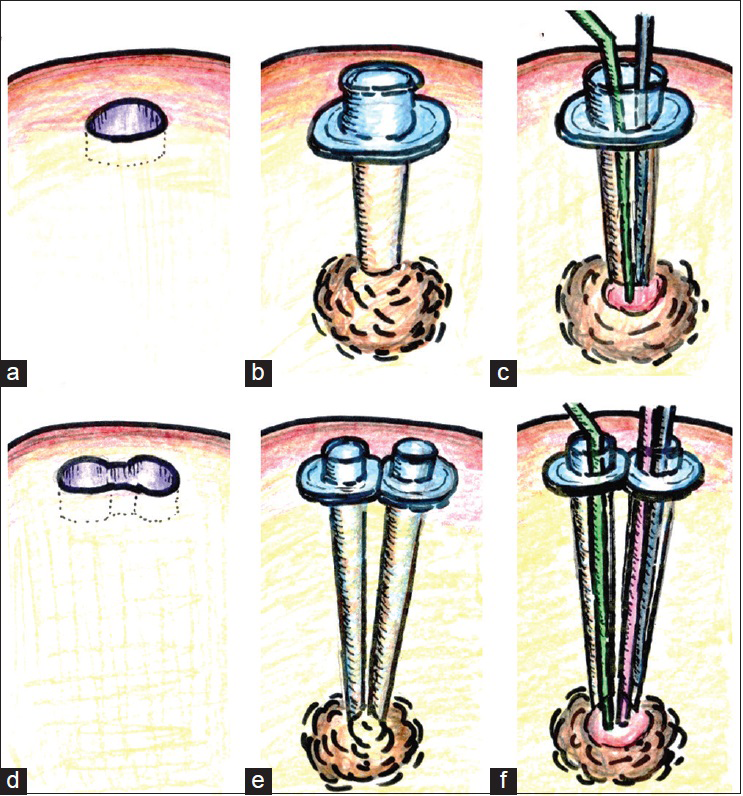
Figure 1
Procedures for rigid endoscopic biopsy using the single port technique (a-c). (a) A round-shaped burr-hole is made. (b) A transparent sheath with diameters of 6.8 mm (or 10.0 mm) is inserted into the front of the target lesion under the control of the navigation system. (c) Observed with a rigid endoscope (a blue column), the lesion is biopsied using a single instrument, such as a biopsy forceps (a green column). Each instrument excepting the rigid endoscope is usually inserted alternately. Procedures for rigid endoscopic biopsy using the dual port technique (d-f). (d) An infinity-shaped burr hole is made. (e) Two transparent sheaths with diameters of 6.8 mm with Nelaton catheters (Fr 18) as alternative inner tubes are inserted into the front of the target lesion under the control of the navigation system. (f) Observed with a rigid endoscope (a blue column), the lesion is biopsied or removed partially using a biopsy forceps (a green column) along with other instrument such as a suction tube (a red column)
RESULTS
The clinical characteristics of the 34 patients with intraparenchymal brain lesions who underwent rigid endoscopic biopsy via the transcortical route are shown in
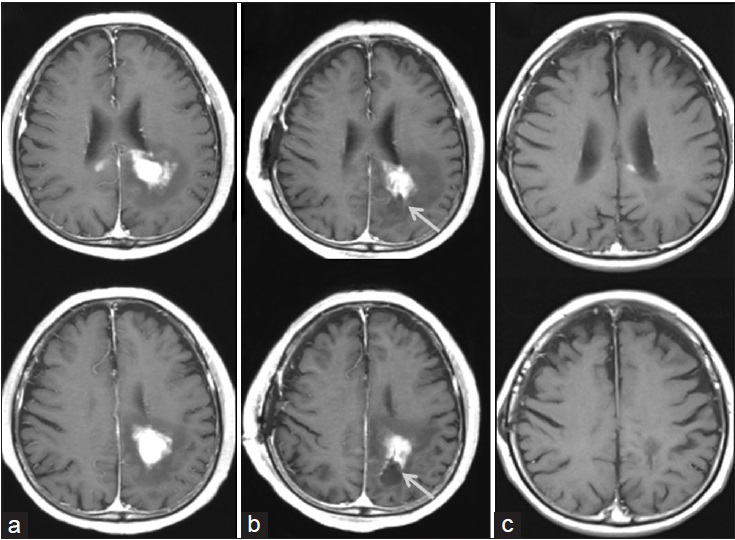
Figure 2
A representative malignant lymphoma case (patient 29) of navigation-guided rigid endoscopic biopsy using the single port technique for the deep white matter lesion of the left parietal lobe. ((a) axial view of T1-weighted images (WI) after gadolinium administration before the biopsy; (b) T1-weighted images-gadolinium 1-day after the biopsy; (c) T1-weighted images-gadolinium after chemotherapy)
The results of the endoscopic biopsy are shown in
There was no postoperative mortality. Only in 2 early cases of this 34-case series, minor postoperative permanent morbidity (5.9%), presumably related to this technique, was observed. One patient with moderate hemiparesis before surgery had worsened hemiparesis probably due to edema and ischemia of the pyramidal tract. Another patient had quadrantanopia due to the injury of the optic tract. Only 2 patients had transient neurological deterioration after the surgery, including 1 patient with transient symptomatic increased bleeding in the residual tumor lesion with mild preoperative intratumoral bleeding, and 1 patient with worsened aphasia due to the enlargement of the biopsy cavity. One patient had complete atrioventricular block after surgery, which was thought to be incidental cardiac trouble independent of the neurosurgical technique. Three cases had asymptomatic minor bleedings in the postresection cavity or in the residual tumor lesion.
DISCUSSION
In the present study, the largest case series among other reports using navigation-guided rigid endoscopic biopsy has been analyzed; with the definitive diagnosis ratio of 97% in 34 continuous cases of patients. In 6 cases, the dual port technique was used to access deeper lesions, resulting in 100% of the definitive diagnosis ratio. Such results were similar to those in smaller case series (100% in 6 cases and 100% in 21 cases) of navigation-guided rigid endoscopic biopsy performed in other institutions.[
We believe that the key characteristics of the ideal biopsy procedure include (1) accurate sampling using a variety of techniques including neuronavigation, intraoperative PDD, and intraoperative pathological diagnosis, (2) sampling a large volume, and (3) visualization of the intraparenchymal structures during surgery. Although open biopsy might be superior to needle biopsy with regards to these key points, both approaches are not always optional for all biopsy cases, including lesions in some deep areas of the white matter, the brain stem and the basal ganglia. We believe that the navigation-guided rigid endoscopic biopsy is a good alternative to other biopsy methods, except for superficial brain lesions, and brain stem lesions.
PDD might be helpful to immediately evaluate whether an accurate sample is obtained from the intraparenchymal brain lesion, including high-grade glioma and malignant lymphoma diagnosis.[
CONCLUSION
We have reported 34 cases of the navigation-guided endoscopic biopsy for intraparenchymal brain lesions. This method was concluded to be safe and feasible for diagnostic tissue sampling.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to thank Thomas Mayers (Medical English Communication Center, Faculty of Medicine, University of Tsukuba) for native English revision.
References
1. Azab WA, Nasim K, Chelghoum A, Parwez A, Salaheddin W. Endoscopic biopsy of brain tumors: Does the technique matter?. Surg Neurol Int. 2014. 5: 159-
2. Bernays RL, Kollias SS, Khan N, Brandner S, Meier S, Yonekawa Y. Histological yield, complications, and technological considerations in 114 consecutive frameless stereotactic biopsy procedures aided by open intraoperative magnetic resonance imaging. J Neurosurg. 2002. 97: 354-62
3. Czyz M, Tabakow P, Weiser A, Lechowicz-Glogowska BE, Zub LW, Jarmundowicz W. The safety and effectiveness of low field intraoperative MRI guidance in frameless stereotactic biopsies of brain tumours-design and interim analysis of a prospective randomized trial. Neurosurg Rev. 2014. 37: 127-37
4. Frati A, Pichierri A, Bastianello S, Raco A, Santoro A, Esposito V. Frameless stereotactic cerebral biopsy: Our experience in 296 cases. Stereotact Funct Neurosurg. 2011. 89: 234-45
5. Grossman R, Nossek E, Shimony N, Raz M, Ram Z. Intraoperative 5-aminolevulinic acid-induced fluorescence in primary central nervous system lymphoma. J Neurosurg. 2014. 120: 67-9
6. Jain D, Sharma MC, Sarkar C, Gupta D, Singh M, Mahapatra AK. Comparative analysis of diagnostic accuracy of different brain biopsy procedures. Neurol India. 2006. 54: 394-8
7. Lu Y, Yeung C, Radmanesh A, Wiemann R, Black PM, Golby AJ. Comparative effectiveness of frame-based, frameless, and intraoperative magnetic resonance imaging-guided brain biopsy techniques. World Neurosurg. 2015. 83: 261-8
8. Masuda Y, Ishikawa E, Takahashi T, Ihara S, Yamamoto T, Zaboronok A. Dual-port technique in navigation-guided endoscopic resection for intraparenchymal brain tumor. Surg Neurol Int. 2012. 3: 35-
9. McGirt MJ, Woodworth GF, Coon AL, Frazier JM, Amundson E, Garonzik I. Independent predictors of morbidity after image-guided stereotactic brain biopsy: A risk assessment of 270 cases. J Neurosurg. 2005. 102: 897-901
10. Nagahisa S, Watabe T, Sasaki H, Nishiyama Y, Hayashi T, Hasegawa M. Surgical navigation-assisted endoscopic biopsy is feasible for safe and reliable diagnosis of unresectable solid brain tumors. Neurosurg Rev. 2013. 36: 595-600
11. Nimsky C, Fujita A, Ganslandt O, von Keller B, Kohmura E, Fahlbusch R. Frameless stereotactic surgery using intraoperative high-field magnetic resonance imaging. Neurol Med Chir (Tokyo). 2004. 44: 522-33
12. Nishihara M, Sasayama T, Kudo H, Kohmura E. Morbidity of stereotactic biopsy for intracranial lesions. Kobe J Med Sci. 2011. 56: E148-53
13. Onuma K, Ishikawa E, Matsuda M, Shibata Y, Satomi K, Yamamoto T. Navigation-guided endoscopic biopsy for pathological diagnosis for intraparenchymal pure germinoma near the ventricular trigone. Surg Neurol Int. 2012. 3: 9-
14. Quinn J, Spiro D, Schulder M. Stereotactic brain biopsy with a low-field intraoperative magnetic resonance imager. Neurosurgery. 2011. 68: 217-24
15. Schucht P, Knittel S, Slotboom J, Seidel K, Murek M, Jilch A. 5-ALA complete resections go beyond MR contrast enhancement: Shift corrected volumetric analysis of the extent of resection in surgery for glioblastoma. Acta Neurochir (Wien). 2014. 156: 305-12
16. Schulder M, Spiro D. Intraoperative MRI for stereotactic biopsy. Acta Neurochir Suppl. 2011. 109: 81-7
17. Tanei T, Nakahara N, Takebayashi S, Hirano M, Nagatani T, Nishihata T. Endoscopic biopsy for lesions located in the parenchyma of the brain: Preoperative planning based on stereotactic methods. Technical note. Neurol Med Chir (Tokyo). 2012. 52: 617-21
18. Tsuda K, Ishikawa E, Zaboronok A, Nakai K, Yamamoto T, Sakamoto N. Navigation-guided endoscopic biopsy for intraparenchymal brain tumor. Neurol Med Chir (Tokyo). 2011. 51: 694-700
19. Widhalm G, Kiesel B, Woehrer A, Traub-Weidinger T, Preusser M, Marosi C. 5-Aminolevulinic acid induced fluorescence is a powerful intraoperative marker for precise histopathological grading of gliomas with non-significant contrast-enhancement. PLoS One. 2013. 8: e76988-
20. Yamada K, Goto S, Kochi M, Ushio Y. Stereotactic biopsy for multifocal, diffuse, and deep-seated brain tumors using Leksell's system. J Clin Neurosci. 2004. 11: 263-7