- Department of Neurosurgery, King Abdullah Medical City, Mecca, Western Province, Saudi Arabia,
- Department of Radiology, King Abdullah Medical City, Mecca, Western Province, Saudi Arabia,
- Department of Pathology, King Abdullah Medical City, Mecca, Western Province, Saudi Arabia,
- Department of Neurosurgery, Cairo University School of Medicine and Teaching Hospitals, Manial, Cairo, Egypt.
Correspondence Address:
Waeel Hamouda
Department of Neurosurgery, Cairo University School of Medicine and Teaching Hospitals, Manial, Cairo, Egypt.
DOI:10.25259/SNI_622_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Sultan Al-Saiari1, Khalid Al-Orabi1, Ahmed Farag1, Zaina Brinji2, Azza Azzouz2, Tahira Mohammed3, Deepa Mushtaq3, Waeel Hamouda4. Intrasellar cavernous hemangiomas: A case report with a comprehensive review of the literature. 17-Feb-2021;12:58
How to cite this URL: Sultan Al-Saiari1, Khalid Al-Orabi1, Ahmed Farag1, Zaina Brinji2, Azza Azzouz2, Tahira Mohammed3, Deepa Mushtaq3, Waeel Hamouda4. Intrasellar cavernous hemangiomas: A case report with a comprehensive review of the literature. 17-Feb-2021;12:58. Available from: https://surgicalneurologyint.com/surgicalint-articles/10595/
Abstract
Background: Extra-axial cerebral cavernous hemangiomas particularly those found in the sellar region, are extremely rare. Their clinical manifestations and imaging characteristics can mimic those of a pituitary adenoma thus making preoperative diagnosis difficult. Few cases are reported in the literature. We present a case, along with a comprehensive review of the literature regarding specific aspects of diagnosis and management of all similarly reported rare cases.
Case Description: We present the clinical, radiological, and operative data of a rare case of a large intrasellar cavernous hemangioma in a 49-year-old female patient presented with headache and diminution of vision, which was diagnosed intraoperatively during an endonasal endoscopic transsphenoidal approach. Subtotal debulking was performed with immediate postoperative clinical improvement. The patient was then referred for radiotherapy and maintained her clinical improvement since then.
Conclusion: Neurosurgeons should consider this rare pathology in the preoperative differential diagnosis of sellar tumors. Bright hyperintense T2 signal with or without signal voids associated with centripetal delayed contrast enhancement in magnetic resonance imaging images might raise the suspicion which can be further confirmed intraoperatively with frozen sections. Due the reported high vascularity and intraoperative profuse bleeding leading to high operative morbidities, piecemeal subtotal resection followed by radiosurgery may be considered today as the safest and most effective strategy.
Keywords: Cavernous hemangioma, Radiosurgery, Sellar, Transsphenoidal
INTRODUCTION
Cerebral cavernous hemangiomas (CCHs) are benign vascular lesions with an incidence of 0.5% among all individuals.[
PRESENTATION OF THE CASE
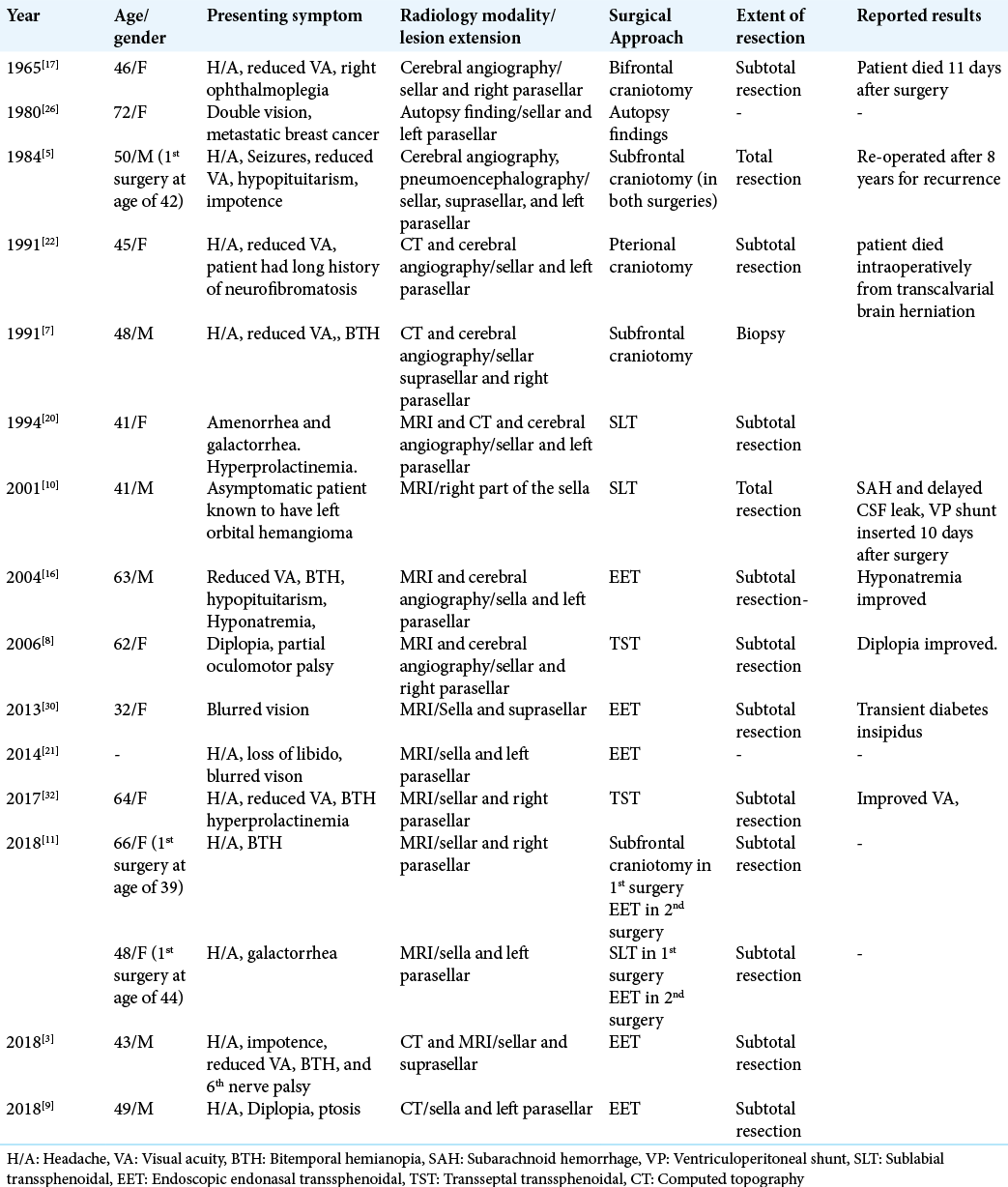
A 49-year-old female was referred to our hospital as a case of a large symptomatizing nonfunctioning pituitary macroadenoma for surgical management. On thorough clinical evaluation, the patient reported that she has been complaining of chronic bitemporal headache, unexplained easy fatiguability, and a very slowly progressive diminution of vision over the previous 2 years. She also reported having high blood pressure which was adequately controlled by medications. She reported no history of any surgical intervention or any familial diseases. On examination, cranial nerves assessment was unremarkable except for her vision problem. Detailed ophthalmological examination revealed decreased visual acuity in both eyes, more on the left, with bitemporal homonymous hemianopia. Endocrinological assessment and biochemical tests revealed central hypothyroidism which was managed by levothyroxine replacement therapy. Computed topography imaging of the brain showed a slightly hyperdense large sellar/suprasellar mass extending to the cavernous sinuses on both sides with the right parasellar extension. The lesion was expanding the bony sella turcica with indentation and remodeling of the upper clivus [
Figure 1:
Preoperative CT images. (a) Sagittal nonenhanced CT scan of the brain demonstrating a large sellar/suprasellar hyperdense lesion with superior extension compressing the floor of the 3rd ventricle and expanding the sella turcica and remodeling the upper clivus. (b) Coronal nonenhanced CT scan of the same lesion demonstrating the parasellar extension of the lesion which is more to the right side.
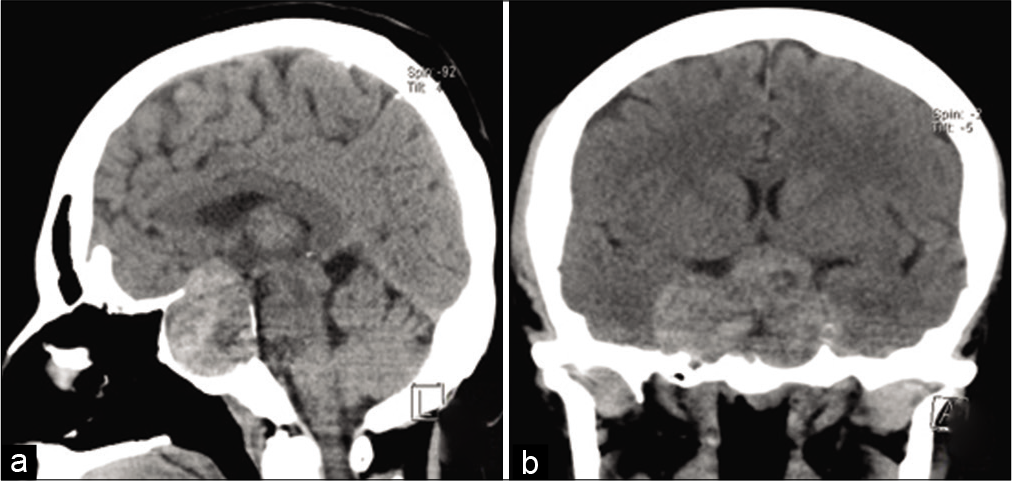
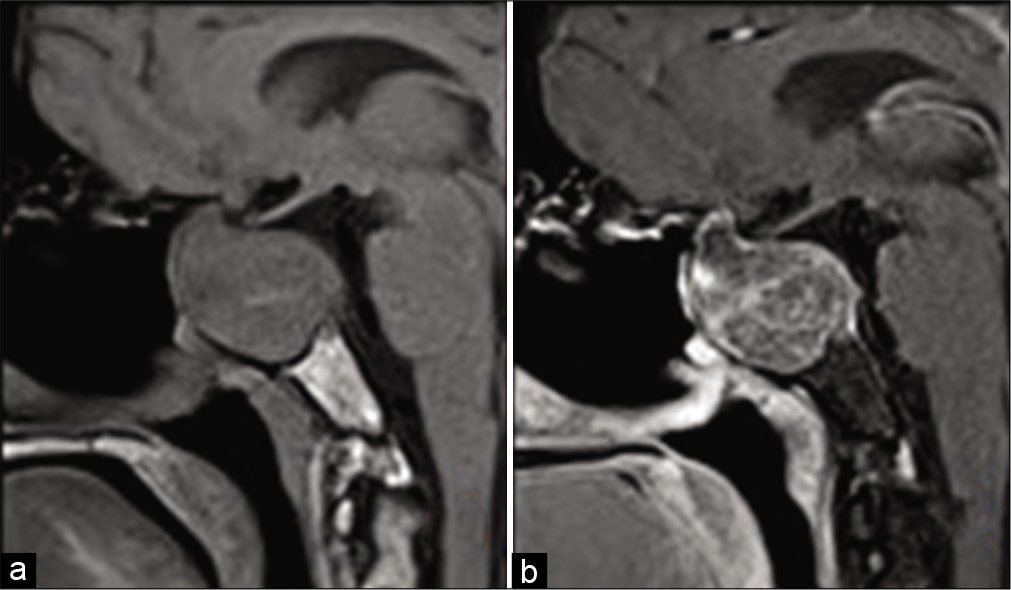
MRI of the sellar area with intravenous contrast administration showed large sellar/suprasellar/parasellar mass, with heterogenous low signal in T1 images, intermediate high signal in T2 images, central small areas of diffusion restriction in DWI images, and mild patchy enhancement in postcontrast images. It measured approximately 36x41x50 mm on anteroposterior, cephalocaudal, and transverse dimensions, respectively. The lesion was completely encasing the cavernous and clinoid segments of the internal carotid arteries on the right side while partially encasing the right supraclinoid and the left cavernous segments. The lesion also exerted mass effect on the adjacent structures in the form of elevation of the tuber cinereum, and the floor of the third ventricle, with the intracranial segments of the optic nerves and optic chiasm being stretched over and inseparable from the lesion. The pituitary gland and the pituitary stalk could not be visualized [
Figure 2:
Preoperative MRI images. (a) Coronal T1, (b) sagittal T1, (c) coronal T2, (d) coronal post contrast, (e) sagittal post contrast, (f) axial DWI images of the lesion showing heterogeneous low T1 signal, intermediate high T2 signal, patchy post contrast enhancement, with central areas of diffusion restriction.
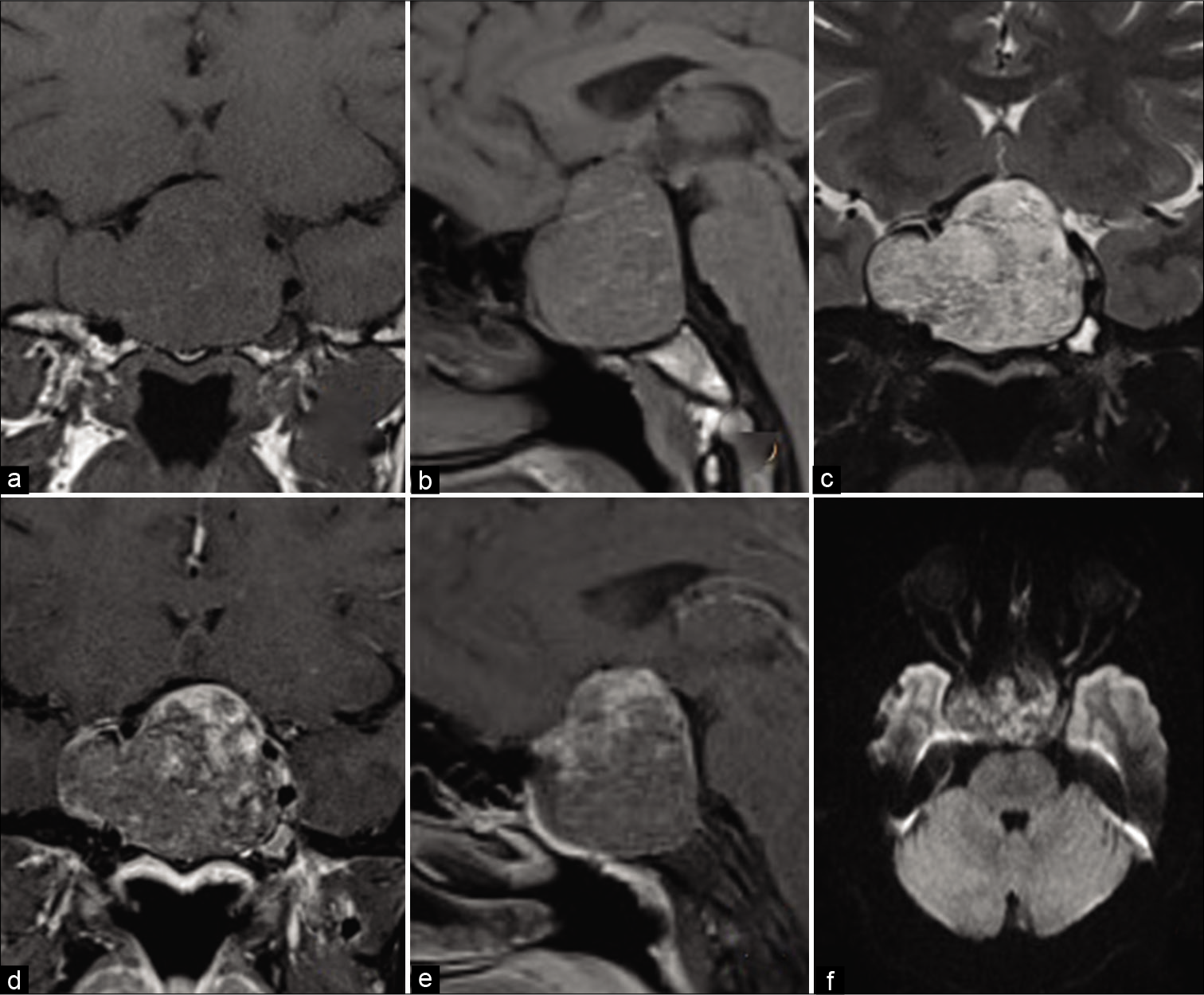
An endoscopic endonasal transsphenoidal (EET) route was used to approach the lesion with the cooperation of a head and neck surgeon. During opening of the sellar floor, a dark red and firm lesion was encountered that seemed to be of vascular nature. Excessive bleeding was encountered on attempt to debulk the lesion. An intraoperative frozen section biopsy confirmed the vascular nature of the lesion and excluded a pituitary pathology. Considering the unknown nature of the lesion, its high vascularity and propensity for bleeding, the decision was to perform a subtotal resection of the lesion aiming for mass effect reduction which was achieved without complications. Special attention was directed to obtain complete hemostasis. A subsequent histopathological examination confirmed the diagnosis of CCH [
Figure 3:
Histopathological slides images. (a and b) Sections from the specimen reveal a neoplasm composed of multiple large vascular channels lined by endothelial cells and separated by loose fibrous tissue with focal lymphocytic infiltrate. Most of them contain red blood cells in their lumina. Few areas of fibrosis and focal whorling is noted. No pituitary gland tissue seen. No evidence of malignancy in the examined biopsy material. (c) CD34 immunohistochemical stain highlighting the small capillaries.
Clinical reassessment during the postoperative period revealed marked improvement of the patient’s visual acuity and field. Follow-up MRI showed adequate decompression of the optic chiasm and nerves despite a large intrasellar residual lesion [
The patient was offered a second stage surgery to attain a more radical excision, but she was satisfied with the improvement in her vision and refused. The case was thoroughly discussed in a multidisciplinary team meeting and the patient was referred for stereotactic radiosurgery. At the follow-up visit after 6 months, the patient is still maintaining her clinical improvement.
DISCUSSION
CCHs were initially classified as rare vascular lesions but have recently become an exceeding finding in multiple locations, especially with recent advances in imaging technology.[
In addition, there are no pathognomonic MRI features that can confidently distinguish these lesions preoperatively.[
Multiple surgical approaches including pterional and subfrontal craniotomies, or sublabial, transseptal, and endoscopic endonasal transsphenoidal approaches have reportedly been utilized for the treatment of such lesions [
As the sellar cavernous hemangioma are vascular lesions and postulated to be dural based,[
Managing residual cavernous hemangioma with adjunctive treatments, such as stereotactic radiosurgery, after confirming the diagnosis with the surgical biopsy can achieve an excellent outcome and avoid additional morbidities.[
CONCLUSION
Sellar cavernous hemangioma is rare vascular lesions that can present with clinical picture and radiological features mimicking pituitary adenomas. Neurosurgeons should consider this rare pathology in the preoperative differential diagnosis of sellar tumors. Bright hyperintense T2 signal with or without signal voids associated with centripetal delayed contrast enhancement in MRI images might raise the suspicion which can be further confirmed intraoperatively with frozen sections. Due the reported high vascularity and profuse bleeding, piecemeal subtotal resection aiming for neural tissues decompression followed by radiotherapy may be considered today as the safest and most effective strategy.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Agha RA, Borrelli MR, Farwana R, Koshy K, Fowler AJ, Orgill DP. The SCARE 2018 statement: Updating consensus surgical case report (SCARE) guidelines. Int J Surg. 2018. 60: 132-6
2. Aiba T, Tanaka R, Koike T, Kameyama S, Takeda N, Komata T. Natural history of intracranial cavernous malformations. J Neurosurg. 1995. 83: 56-9
3. Al-Sharydah AM, Al-Suhibani SS, Al-Jubran SA, AlAbdulwahhab AH, Al-Bar M, Al-Jehani HM. Endoscopic management of Atypical sellar cavernous hemangioma: A case report and review of the literature. Int J Surg Case Rep. 2018. 42: 161-4
4. Batra S, Lin D, Recinos PF, Zhang J, Rigamonti D. Cavernous malformations: Natural history, diagnosis and treatment. Nat Rev Neurol. 2009. 5: 659-70
5. Buonaguidi R, Canapicci R, Mimassi N, Ferdeghini M. Intrasellar cavernous hemangioma. Neurosurgery. 1984. 14: 732-4
6. Chapman PR, Gaddamanugu S, Bag AK, Roth NT, Vattoth S. Vascular lesions of the central skull base region. Semin Ultrasound CT MR. 2013. 34: 459-75
7. Chhang WH, Khosla VK, Radotra BD, Kak VK. Large cavernous haemangioma of the pituitary fossa: A case report. Br J Neurosurg. 1991. 5: 627-9
8. Chibbaro S, Cebula H, Ganau M, Gubian A, Todeschi J, Lhermitte B. Multidisciplinary management of an intrasellar cavernous hemangioma: Case report and review of the literature. J Clin Neurosci. 2018. 52: 135-8
9. Chuang CC, Jung SM, Yang JT, Chang CN, Pai PC. Intrasellar cavernous hemangioma. J Clin Neurosci. 2006. 13: 672-5
10. Cobbs CS, Wilson CB. Intrasellar cavernous hemangioma. Case report. J Neurosurg. 2001. 94: 520-2
11. Das S, Ang LC, Ramsay D. Intrasellar cavernous hemangioma presenting as pituitary adenoma: A report of two cases and review of the literature. Clin Neuropathol. 2018. 37: 64-7
12. Flemming KD, Lanzino G. Stereotactic radiosurgery for cavernous malformations: Natural history or treatment effect?. Neurology. 2019. 93: 921-2
13. Flemming KD. Clinical management of cavernous malformations. Curr Cardiol Rep. 2017. 19: 122
14. Goldstein HE, Solomon RA. Epidemiology of cavernous malformations. Handb Clin Neurol. 2017. 143: 241-7
15. Hayashi Y, Tachibana O, Mohri M, Kinoshita M, Hayashi Y, Yoshinaga T. Uncommon co-localization of pituitary adenoma and parasellar cavernous angioma. Eur J Radiol Extra. 2008. 66: e1-3
16. Jeon SC, Yi JS, Yang JH, Lee I. Intrasellar cavernous hemangioma. J Korean Neurosurg. 2004. 36: 163-5
17. Kamrin RB, Buchsbaum HW. Large vascular malformations of the brain not visualized by serial angiography. Arch Neurol. 1965. 13: 413-20
18. Kanaan I, Jallu A, Alwatban J, Patay Z, Hessler R. Extra-axial cavernous hemangioma: Two case reports. Skull Base. 2001. 11: 287-95
19. Kondziolka D, Lunsford LD, Kestle JR. The natural history of cerebral cavernous malformations. J Neurosurg. 1995. 83: 820-4
20. Lombardi D, Giovanelli M, de Tribolet N. Sellar and parasellar extra-axial cavernous hemangiomas. Acta Neurochir (Wien). 1994. 130: 47-54
21. Ma LC, Li WY, Chen WQ, Wu YK. Intrasellar cavernous hemangioma. Neurol India. 2014. 62: 95-6
22. Mitsuhashi T, Hashimoto R, Nagahama S, Nagata Y. Intrasellar cavernous angioma in neurofibromatosis. Hum Pathol. 1991. 22: 623-4
23. Nagy G, Kemeny AA. Stereotactic radiosurgery of intracranial cavernous malformations. Neurosurg Clin N Am. 2013. 24: 575-89
24. Poorthuis MH, Klijn CJ, Algra A, Rinkel GJ, Salman RA. Treatment of cerebral cavernous malformations: A systematic review and meta-regression analysis. J Neurol Neurosurg Psychiatry. 2014. 85: 1319-23
25. Poorthuis MH, Rinkel LA, Lammy S, Al-Shahi Salman R. Stereotactic radiosurgery for cerebral cavernous malformations: A systematic review. Neurology. 2019. 93: E1971-9
26. Sansone ME, Liwnicz BH, Mandybur TI. Giant pituitary cavernous hemangioma: Case report. J Neurosurg. 1980. 53: 124-6
27. Suzuki M, Matsui O, Ueda F, Matsushita T, Fujinaga Y, Kobayashi K. Dynamic MR imaging for diagnosis of lesions adjacent to pituitary gland. Eur J Radiol. 2005. 53: 159-67
28. Taslimi S, Modabbernia A, Amin-Hanjani S, Barker FG, Macdonald RL. Natural history of cavernous malformation: Systematic review and meta-analysis of 25 studies. Neurology. 2016. 86: 1984-91
29. Tokunaga K, Date I. Clinical features and management of cavernous and venous angiomas in the head. Brain Nerve. 2011. 63: 17-25
30. Turan A, Unlu HA, Yigit H, Yilmaz O, Yakut ZI. Intrasellar cavernous hemangioma: MRI findings of a very rare lesion. J Med Cases. 2013. 4: 719-21
31. Wolfsberger S, Ba-Ssalamah A, Pinker K, Mlynárik V, Czech T, Knosp E. Application of three-tesla magnetic resonance imaging for diagnosis and surgery of sellar lesions. J Neurosurg. 2004. 100: 278-86
32. Wu X, Yu H, Zhao G, Wang L, Liu Y, Li Y. Intrasellar cavernous hemangioma: A case report and literature review. Transl Neurosci Clin. 2017. 3: 111-5