- Department of Neurosurgery, King George’s Medical University, Lucknow, Uttar Pradesh, India.
Correspondence Address:
Manish Jaiswal, Department of Neurosurgery, King George’s Medical University, Lucknow, Uttar Pradesh, India.
DOI:10.25259/SNI_825_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Sarita Kumari, Manish Jaiswal, Bal Krishna Ojha. Is basal cisternostomy in traumatic brain injury a need of hour or white elephant – A randomized trial to answer. 01-Dec-2023;14:412
How to cite this URL: Sarita Kumari, Manish Jaiswal, Bal Krishna Ojha. Is basal cisternostomy in traumatic brain injury a need of hour or white elephant – A randomized trial to answer. 01-Dec-2023;14:412. Available from: https://surgicalneurologyint.com/surgicalint-articles/12653/
Abstract
Background: Basal cisternostomy (BC) recently emerged as an adjuvant/alternative procedure to decompressive craniectomy (DC) in traumatic brain injuries (TBIs) with its potential to effectively reduce both intracranial pressure (ICP) and brain edema. However, its role in TBI is not yet established in the true sense and with clarity. The objective of the present study was to evaluate the effect of adjuvant BC on ICP, mortality, and clinicoradiological outcome.
Methods: A single-center randomized control trial was conducted. Fifty patients were assigned to each DC-group and DC+BC-group. Randomization was done using the sealed envelope method. Both groups were followed in the postoperative period to compare the impact of surgery on ICP, radiological changes, and clinical outcome (mortality, days on ventilator/in intensive care unit (ICU), and Glasgow outcome scale-extended (GOS-E) at 12 weeks).
Results: Both groups were comparable in terms of preoperative clinicoradiological characteristics. On postoperative days 1, 2, and 3, mean ICP was significantly low in the DC+BC-group (P P
Conclusion: Results of our study indicated that BC is beneficial in reducing both ICP and brain edema, which translates into favorable clinicoradiological outcomes.
Keywords: Basal cisternostomy, Intracranial pressure, Traumatic brain injury
INTRODUCTION
Traumatic brain injury (TBI) is one of the leading causes of morbidity and mortality, especially in the younger age group,[
MATERIALS AND METHODS
This was a single-center randomized controlled study conducted at the Department of Neurosurgery, King George’s Medical University, Lucknow, Uttar Pradesh, India, from February 2022 to April 2023. The study was approved by the Institutional Ethical Committee (Registration no: ECR/262/ Inst/UP/2013/RR-19). The study was also registered with the Central Trial Registry of India (CTRI reference no: CTRI/2022/04/042144).
Inclusion criteria
Following were the inclusion criteria.
Patients of age ≥18 years presenting with TBI who were planned for DC (as per institutional protocol) and whose brain was bulging even after evacuation of the traumatic lesion (hematoma and contused brain).
We considered it a brain bulge when the brain surface remained above the level of the inner table of the skull bone of the craniectomy site after eliminating the effect of gravity [
Our institutional clinicoradiological criteria for planning DC in TBI were the followings:
Acute subdural hematoma (SDH) with a maximum thickness of ≥ 10 mm or mass effect and midline shift >5 mm in computed tomography (CT) head irrespective of Glasgow coma scale (GCS) score. Patients with GCS ≤ 9 having acute SDH thickness <10 mm and midline shift of <5 mm whose GCS score decreased by two or more points after hospital admission. Patients with GCS score of 6–8 and frontal and/or temporal contusions of >20 cc in volume with mass effect and midline shift > 5 mm or cisternal compression on CT head. Patients with any supratentorial contusion >50 cc in volume on CT head irrespective of GCS score.
Exclusion criteria
Exclusion criteria were the following: (1) Hemodynamic instability, (2) pregnant females, (3) coagulopathy, (4) brain stem dysfunction and signs of irreversible brain damage (B/L fixed dilated pupils), (5) GCS score 3, (6) acute infarcts with mass effect, (7) extradural hemorrhage/chronic SDH/ posterior fossa bleed, (8) penetrating brain injuries/brain matter leak, (9) non-traumatic subarachnoid hemorrhage (SAH)/intraparenchymal bleeds, and (10) patients for whom consent could not be obtained.
At admission, patients received treatment as per institutional protocol, and data related to trauma was collected. GCS and pupillary reaction were examined at admission and just before surgery. Non-contrast computed tomography (NCCT) head was done. The following details were noted: (1) Side and site of contusion/hematoma, (2) presence or absence of traumatic SAH and intracerebral hemorrhage (IVH), (3) status of cisternal space, (4) midline shift, and (5) presence of ischemia/infarct. Rotterdam scoring was calculated. Patients were divided into three classes – severe, moderate, and mild TBI.
Randomization
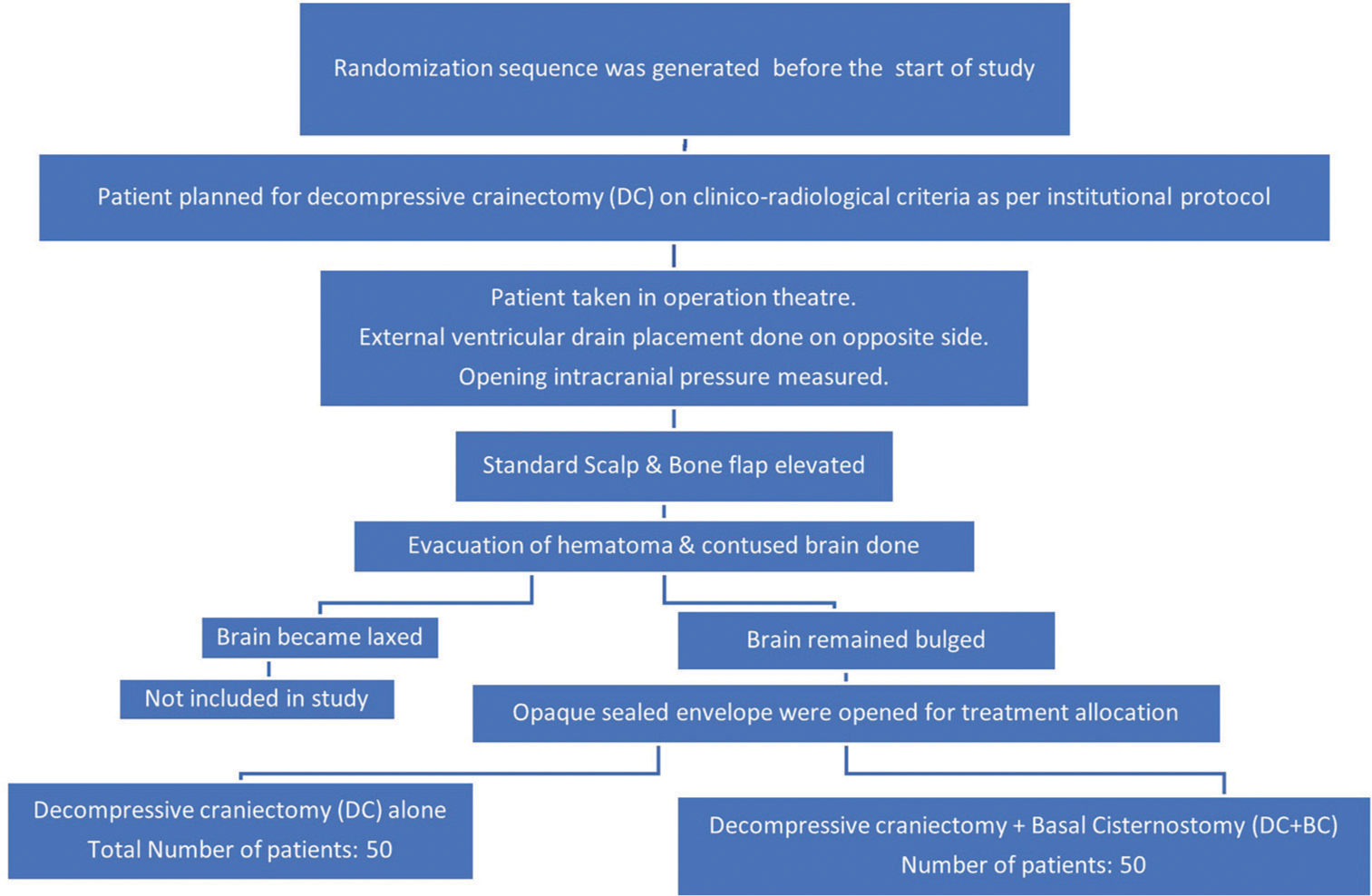
The randomization sequence was generated before the start of the study by a computer-generated set of random numbers. Treatment allocation was done by the opaque sealed envelope method opened by the operation theater (OT) nurse in charge [
Two groups
A total of 100 patients were included in the study. Fifty patients were assigned to the DC group alone and 50 patients to the DC+BC group. Written informed consent from family members of each patient included in the study was taken.
Surgical steps for DC Group
Surgeries were performed under general anesthesia. External ventricular drain (EVD) placement was done on the opposite side of the surgical site for measuring ICP (Not used for draining cerebrospinal fluid (CSF) in either group).
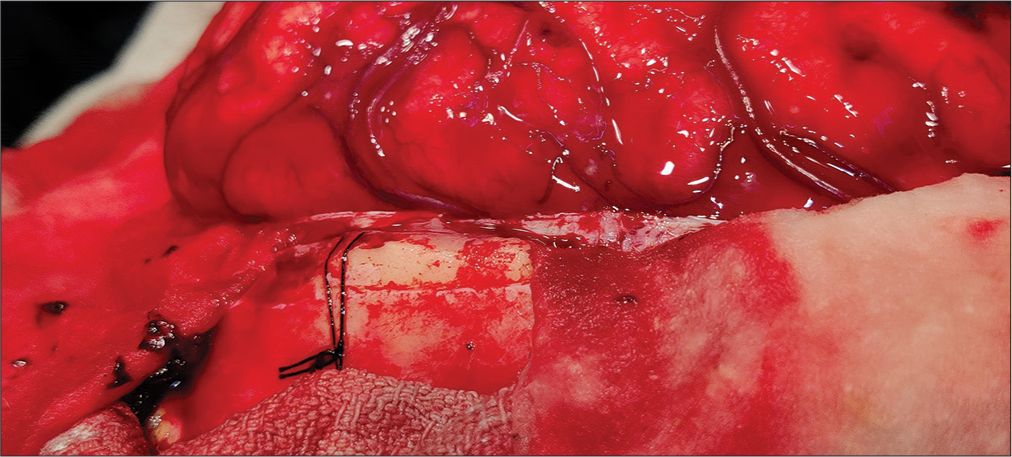
The patient was positioned supine with the head rotated approximately 20–30° toward the opposite side. A standard question mark or reverse question mark bone-deep incision was used. The scalp flap, along with the periosteum, was elevated off the skull and reflected anteriorly. Temporalis muscle was reflected anteroinferiorly. A standard craniotomy of 15 cm × 12 cm was obtained. The temporal bone is nibbled to obtain a craniotomy that is flush with the middle fossa. The sphenoid ridge was cut to the lateral edge of the superior orbital fissure using Kerrison rongeur to visualize the orbitomeningeal fold and artery. The orbitomeningeal band was cut to unfurl the frontal and temporal dura. After that, the dura was opened in a C-shaped manner, and hematoma (Acute SDH/contusion) evacuation was done. After that, standard lax duraplasty was done with autologous pericranium. Temporalis muscle was sutured, followed by skin closure, which was done in two layers (galea and skin). The bone flap was placed in a subcutaneous pouch made in the anterior abdominal wall.
Surgical steps for DC+BC group
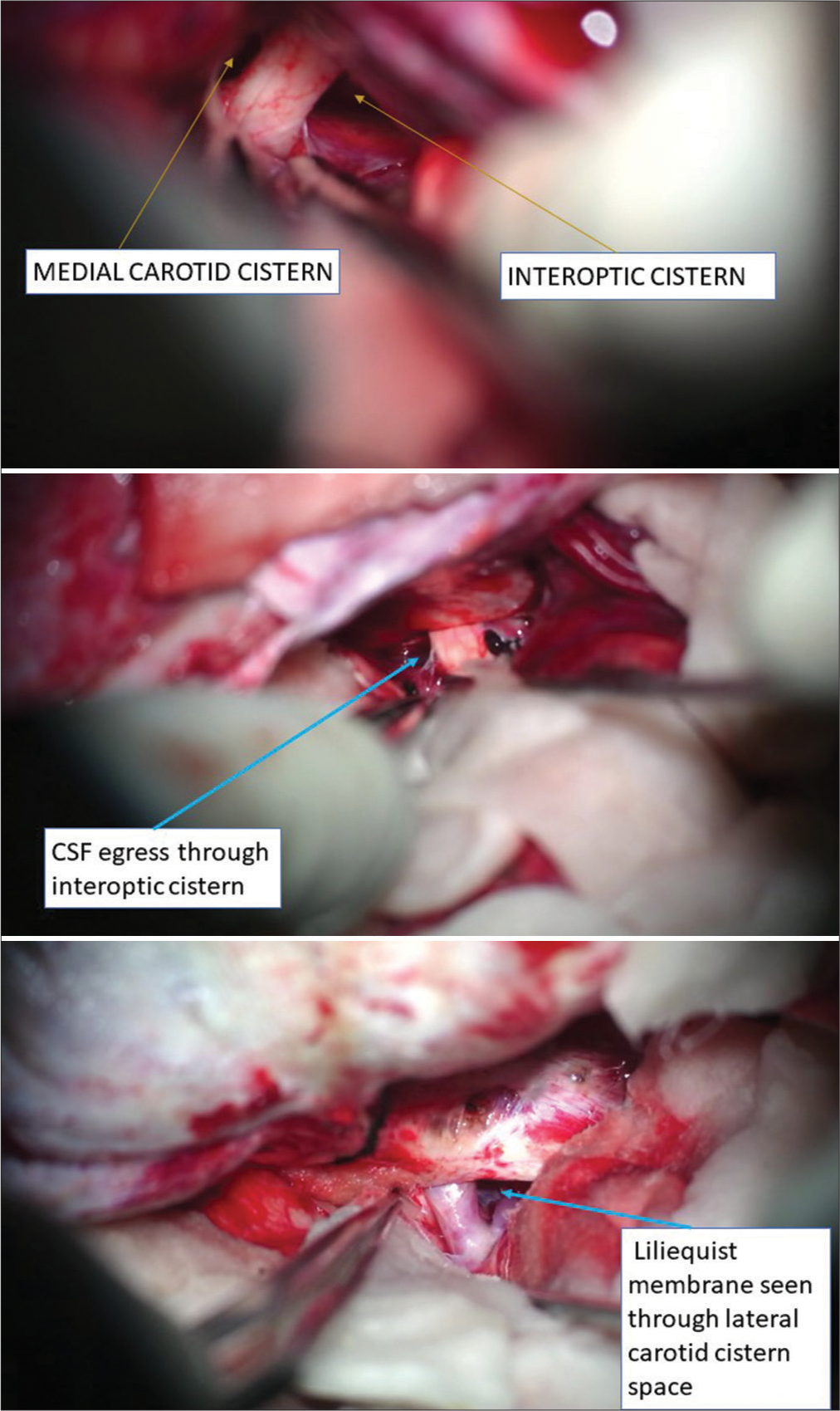
All steps till the evacuation of hematoma were similar to the DC group. After the evacuation of the hematoma, a lateral subfrontal approach was used to reach basal cisterns. The frontal lobe was retracted gently, and the olfactory tract was identified. We used dynamic retraction with the help of a suction cannula of small caliber and bayonet-shaped long micro forceps in place of a fixed retraction system. Inter-optic, optico-carotid, and lateral carotid cisterns were identified and opened. Liliequist membrane was perforated through the optico-carotid window or the lateral carotid window to open interpeduncular and prepontine cisterns, and the basilar artery is visualized [
Postoperative neurocritical care, ICP monitoring, and outcome assessment
Patients of both groups were shifted to the intensive care unit (ICU) after the surgery for standard neurocritical care management. ICP monitoring was done for 72 h in the postoperative period with the help of EVD. The EVD was kept in situ for 72 h and used exclusively for measuring ICP. CSF drainage through EVD was not done in either group to avoid its lowering effect on ICP and its confounding effect on the result. All patients were sedated and mechanically ventilated, aiming to keep PaO2 and PaCO2 between 90 and 100 mmHg and 36 and 40 mmHg, respectively. Cerebral perfusion pressure was maintained between 60 and 70 mmHg with the use of isotonic fluids and vasopressors. Metabolic control included the maintenance of normoglycemia and normothermia. In our study, we did not place PbtO2 probes due to constraints of resources. If the ICP reading remained superior to 20 mmHg for more than 5 min, osmotherapy consisting of intravenous bolus (over 20 min) of 20% mannitol (0.5 g/kg) was administered. The numbers of osmotherapy bolus required in both groups were calculated separately for comparison. Two groups were compared for the total number of days in ICU/on mechanical ventilation, the in-hospital mortality rate (related to TBI sequelae), GCS, and GOS-E at 12 weeks follow-up. Postoperative CT scans were done on postoperative days 0, 3, and 7 or as and when required. Midline shift and brain outward herniation were noted. Postoperative complication (clinical/radiological) was also noted down.
Statistical analysis
Collected data with proper headings were entered in a Microsoft Excel 2019 (Microsoft Corporation, Redmond, Washington, USA) datasheet. Data were expressed as mean ± standard deviation for continuous variables. Data were represented as counts and percentages for categorical variables. Student t-test was used to make a comparison of means between 2 groups, in case data were normally distributed; else, the Mann–Whitney U-test was used. The chi-square test was used to compare categorical variables. For statistical analysis, IBM SPSS Version 21.0 (IBM, Corporation, Armonk, New York, USA) was used.
RESULTS
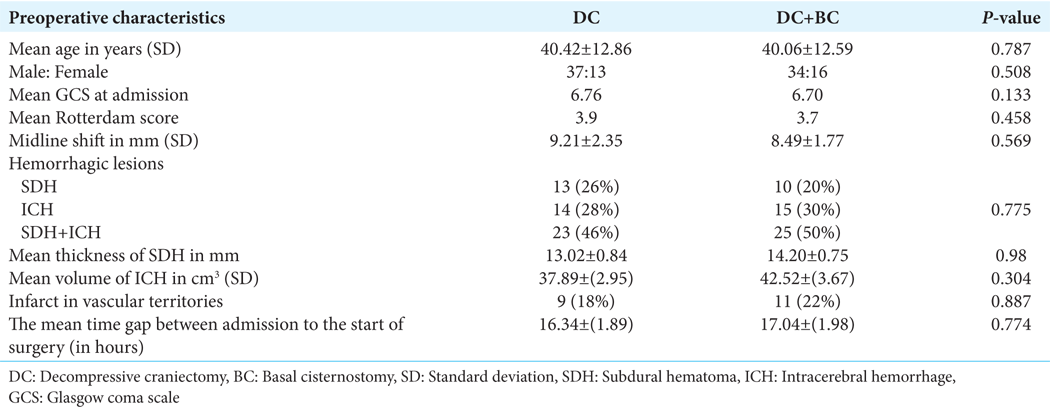
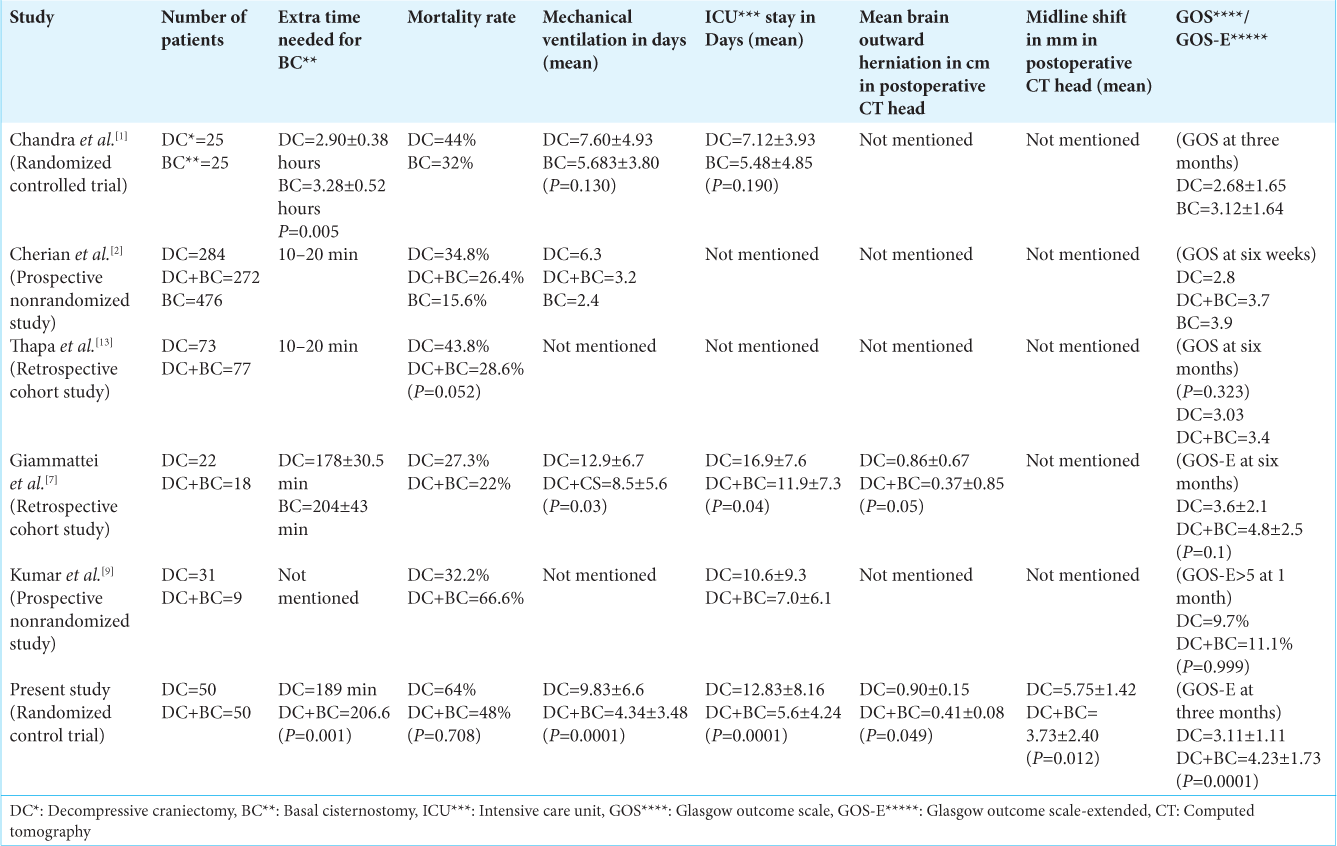
A total of 100 patients who fulfilled the inclusion criteria were included in the study. Most of the patients in both groups were in low GCS (4–8) at the time of admission. In the DC group, 37 patients, and in the DC+BC-group, 43 patients had severe TBI. A total of 13 patients in the DC group and seven patients in the DC+ BC-group had moderate TBI. No patient in either group had mild TBI. On data analysis, the two groups were homogenous for preoperative clinicoradiological characteristics (age and sex, duration between trauma and admission, GCS at admission, type and size/volume of hemorrhage, Midline shift, status of cisternal space, presence of ischemia/infarct in vascular territories, and Rotterdam score in preoperative NCCT head) [
Duration of surgical procedures
The mean duration of surgery was 189 min in the DC group and 206.6 min in the DC+BC group (P < 0.0001*). Hence, on average, the extra time needed to perform cisternostomy was approximately 15–20 min. The bone flap was replaced at the craniotomy site during primary surgery in ten patients out of 50 patients randomized in the DC+BC group.
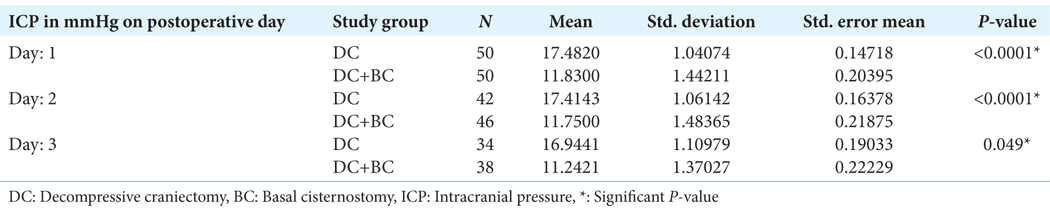
Intracranial pressure
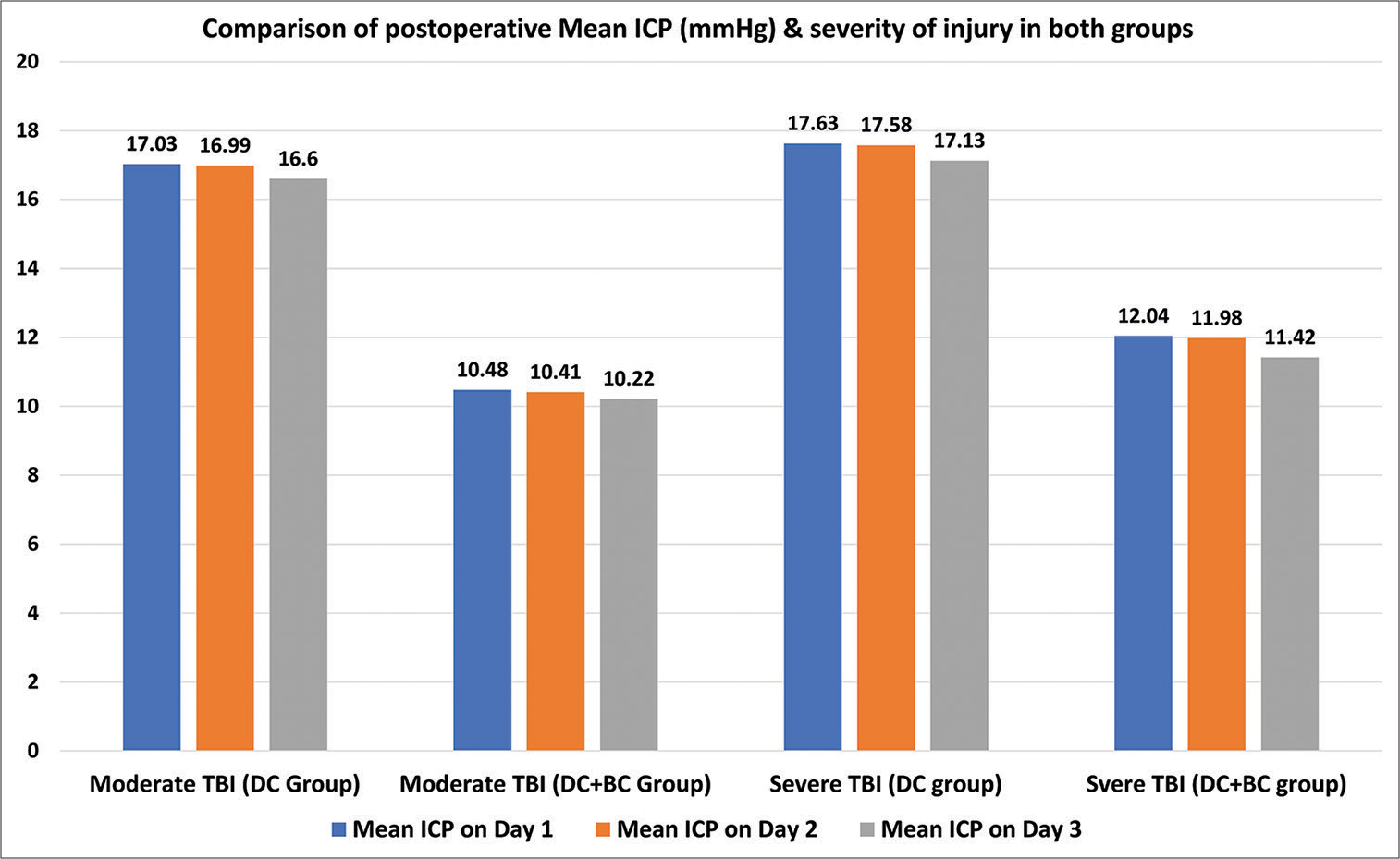
The mean opening intracranial pressure was 26.52 ± 1.18 mm of Hg in the DC group and 27.46 ± 1.13 mm of Hg in the DC-BC group (P = 0.773, which was insignificant). Mean ICP was significantly low in DC+BC group on postoperative day-1, day-2, and day-3 as compared to DC group having P < 0.0001*, P < 0.0001*, and P < 0.049*, respectively on day-1, day-2, and day-3 [
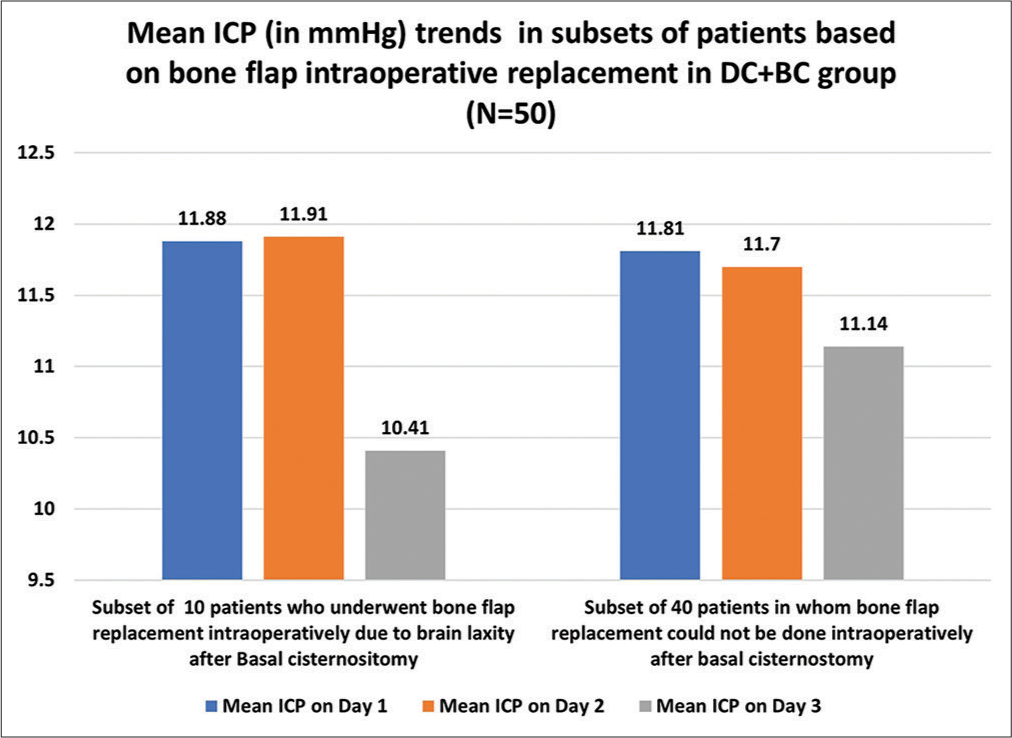
We further analyzed the mean ICP trends in two subsets in the DC+BC group, a first subset of ten patients who underwent bone flap replacement intraoperatively due to sufficient brain laxity after BC and a second subset of 40 patients in whom bone flap could not be replaced. We found no statistical difference in mean ICP in these two subsets on day one and day 2 (P = 0.638 and 0.734, respectively). Still, it was significantly low on day 3 in the first subset of ten patients as compared to a second subset of the DC+BC group (P = 0.045*) [
Osmotherapy requirement
In the postoperative period, patients in the DC group required osmotherapy boluses more frequently. The total number of times boluses of i/v 20% Mannitol needed in the DC group was 266 as compared to 112 times in the DC+BC group. Consequently, bolus osmotherapy was delivered in the DC group at a rate of 5.3 per patient, compared to 2.2 in the DC+BC group (P = 0.02*).
Clinical and radiological outcome
In our study, the in-hospital mortality rate in the DC group was 64 %, and the DC+BC group was 48%. Although there is a difference in percentages, this difference was not clinically significant (P = 0.708). Overall, in-hospital mortality in our study was 56%. The duration of mechanical ventilation required in the DC group was 9.83 ± 6.6 days, and in the DC+BC group was 4.34 ± 3.48 days (P < 0.0001*). The number of days required in ICU was 12.83 ± 8.16 days in the DC group and 5.6 ± 4.24 days in the DC+BC group (P < 0.0001*). The calculated mean GCS at 12 weeks was 13.03 ± 0.94 for the DC group and 14.3 ± 0.66 for the DC+BC group (P = 0.196). Mean GOS-E at 12 weeks was 3.11 ± 1.11 for the DC group and 4.23 ± 1.73 for the DC+BC group (P < 0.0001*). On further subgroup analysis based on severity of head injury at the time of admission, we observed that GOS-E at 12 weeks was 3.5 ± 1.23 in moderate TBI-DC subgroup and 2.8 ± 1.01 in the severe TBI-DC subgroup, whereas it was 4.57 ± 1.78 in moderate TBI DC+BC subgroup and 4.10 ± 1.67 in severe TBI DC+BC subgroup. No patient in the DC group attained a favorable outcome at six weeks, whereas 11 (22%) patients in the DC+BC group were in the favorable outcome category. All these 11 patients belonged to lower moderate disability (GOS-E-5).
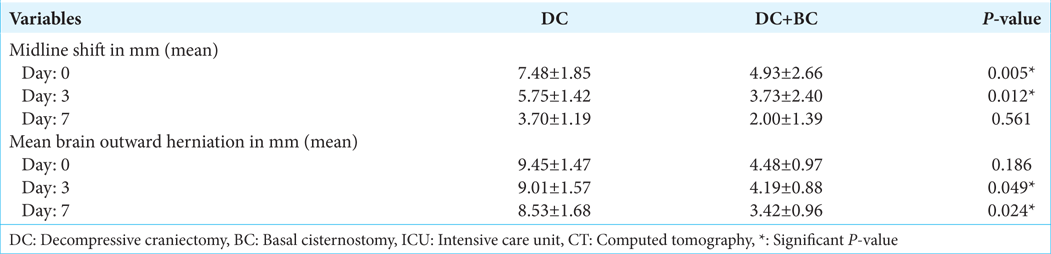
The mean midline shift on postoperative days and 3 in the DC+BC group were significantly less as compared to the DC group, while mean brain outward herniation on postoperative day 3 and 7 in the DC group was statically more as compared to the DC+BC group and in DC+BC group [
Complications
Newer infarcts (venous and arterial) in the brain developed in eight patients of the DC group and two patients of the DC+BC group. IVH developed in three patients in the DC group and two patients in the DC+BC group. SDH developed in one patient in each group. In the DC group, one patient developed subdural hygroma, and one patient developed epidural hematoma (EDH). Three patients in the DC group developed posttraumatic hydrocephalus in a follow-up period of 3 months.
DISCUSSION
The current standard surgical treatment for TBI is decompressive craniectomy. The decompressive craniectomy procedure has proven its role in decreasing ICP and mortality. Whether this translates into a favorable or unfavorable outcome is still under debate. Decompressive craniectomy procedure has its own set of complications, such as external cerebral herniation, blooming of contusions, subdural/EDH or hygroma, strangulation of cerebral tissue at edge of bone flap causing infarction, hydrocephalus, and syndrome of trephine. The pathophysiological basis for these complications is based on the fact that DC helps in reducing ICP but not intracerebral pressure, causing alterations in compliance, cerebral blood flow, autoregulation, and disruption of the subarachnoid CSF pathways and circulation.
Cisternostomy has been recently proposed as an alternative or adjunctive technique that has the potential to reduce ICP as well as brain edema. It is based on the concept of reversal of “CSF shift edema,” the rationale of which lies in recognition of paravascular Virchow Robin spaces that constitute glymphatic pathways and have an important contribution to CSF circulation.[
It is postulated that in TBI, this glymphatic system gets impaired due to traumatic SAH that clogs natural CSF pathways, causing a rise in cisternal pressure, leading to a decrease in interstitial fluid drainage and, hence, brain edema.[
Study design and timing of randomization
Our study was a randomized controlled study, and we decided to do randomization intraoperatively. As we know, in some patients who are being planned for decompressive craniectomy (DECRA), on the basis of clinicoradiological criteria, the brain gets lax after the evacuation of hematoma/contusion. Therefore, they no longer need DECRA. In studies related to BC available in literature till now, patients were selected for BC procedure preoperatively based on only clinicoradiological criteria for DECRA. In these studies, the status of the brain (lax/bulge) was also not clearly defined before performing the cisternostomy procedure. Selection of cases in such a way without considering the effect of hematoma evacuation on brain laxity may lead to a false-positive effect of BC. That’s why we have randomized the patient during the intraoperative period to evaluate the real effect of this procedure.
Surgical technique
Performing BC does require a surgeon skilled in skull base and micro neurosurgery. It becomes even trickier in an edematous brain in the setting of traumatic brain injury. It also needs an OT equipped with an operative microscope. The basal cisternostomy procedure in our study was performed by a single surgeon (corresponding author) experienced in skull base and aneurysm surgery. In studies conducted by Cherian et al.,[
Timing of opening of cisterns
In studies conducted by Cherian et al.,[
No placement of a cisternal drain
In studies conducted by Cherian et al.[
Replacement of bone flap at craniotomy site
Another important aspect of the studies mentioned above was that the brain became lax enough after completing the surgical procedure, so authors were able to replace bone flaps at the craniotomy site in primary surgery in a significant number of cases. There is a possibility that the brain became lax due to evacuation of hematoma/contusion, and whether these candidates were real candidates for DECRA was not clearly mentioned. In our study, in all 50 patients of the DC+BC group, brain bulges decreased to some extent. Still, we were able to observe satisfactory brain laxity in only ten patients in whom the bone flap was replaced at the craniotomy site during primary surgery.
Duration of surgical procedures
Cherian et al.[
Effect on ICP
In a study conducted by Giammattie et al.,[
In our present study, opening pressure was measured intraoperatively and was found to be comparable in two groups. ICP was measured in the postoperative period for 72 h, and the mean postoperative ICP on day 1, day 2, and day three was found to be decreased in both groups. This decline in ICP was more in the DC+BC group as compared to the DC group, and the difference was statistically significant. This result is consistent with the rest of the studies. However, in contrast to other studies, in our study, this significant decrease in ICP in the DC+BC group is not due to CSF drainage through the cisternal drain. Therefore, this decrease in ICP in our study can be attributed to the addition of BC procedure. On subgroup analysis, we observed that adding BC to DC leads to a further decline in ICP in both moderate and severe TBI patients as compared to the DC alone, proving its efficacy in both categories of patients.
On subset analysis, we observed that mean ICP trends were a little higher on day one and day two and lower on day 3 in ten patients who underwent bone flap replacement intraoperatively in the DC+BC group as compared to the rest of the 40 patients’ subset. A possible explanation for slightly higher (but statistically insignificant) mean ICP on day one and day two would be the added bone flap volume, which was replaced at the operative site in these ten patients. Decreasing mean ICP trends on day 3 in this ten patient’s subset might be due to the early establishment of normal physiological CSF flow circulation at brain convexity and its further flow for absorption at arachnoid granulation located in superior sagittal sinus with the help of replaced bone flap of at operative site. As these two subsets are not balanced in terms of patient number, we need further study in the future focusing on these two subsets only to validate our findings.
Effect on radiological outcome
In a study conducted by Giammattei et al.,[
Effect on clinical outcome
In studies conducted by Cherian et al.,[
In studies conducted by Cherian et al.,[
Cherian et al.[
In studies conducted by Giammattei et al.[
Limitations
Our study has certain limitations. It was a single-center study. Patients less than 18 years old and pregnant females were not included in the study. Patients were followed up for three months due to paucity of study time.
CONCLUSION
Adding BC is effective in reducing both ICP as well as brain edema, which translates into clinically relevant patient outcomes. This procedure also has the potential for replacement of bone flap during primary surgery and, hence, can avoid complications of DC and second surgery in the form of cranioplasty. In our study, we also observed that it is a safe and feasible procedure to be performed in a trauma setting as it can be performed without the need for clinoid drilling. With all these, it seems like a promising procedure. Still, the key roadblock is the mandate of surgeons skilled in skull base and micro neurosurgery and the requirement of the operative microscope in neurotrauma setup. The results of our single-center RCT are encouraging, and we recommend that more multi-center trials should be conducted to establish the role of this procedure in trauma settings.
Ethical approval
The author(s) declare that they have taken the ethical approval from IEC (Registration no: ECR/262/ Inst/UP/2013/RR-19).
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Chandra VR, Prasad BC, Banavath HN, Reddy KC. Cisternostomy versus decompressive craniectomy for the management of traumatic brain injury: A randomized controlled trial. World Neurosurg. 2022. 162: e58-64
2. Cherian I, Yi G, Munakomi S. Cisternostomy: Replacing the age old decompressive hemicraniectomy?. Asian J Neurosurg. 2013. 8: 132-8
3. Cherian I, Grasso G, Bernardo A, Munakomi S. Anatomy and physiology of cisternostomy. Chin J Traumatol. 2016. 19: 7-10
4. Cherian I, Burhan H, Dashevskiy G, Motta SJ, Parthiban J, Wang Y. Cisternostomy: A timely intervention in moderate to severe traumatic brain injuries: Rationale, indications, and prospects. World Neurosurg. 2019. 131: 385-90
5. Cherian I, Burhan H. Outcomes of severe head injury patients undergoing Cisternostomy from a tertiary care hospital in Nepal. Indones J Neurosurg. 2019. 2: 55-9
6. Cherian I, Munakomi S. Surgical technique for cisternostomy: A review. Int J Stud Res. 2013. 3: 5
7. Giammattei L, Starnoni D, Maduri R, Bernini A, Abed-Maillard S, Rocca A. Implementation of cisternostomy as adjuvant to decompressive craniectomy for the management of severe brain trauma. Acta Neurochir (Wien). 2020. 162: 469-79
8. Iliff JJ, Chen MJ, Plog BA, Zeppenfeld DM, Soltero M, Yang L. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J Neurosci. 2014. 34: 16180-93
9. Kumar P, Goyal N, Chaturvedi J, Arora RK, Singh PR, Shakya J. Basal cisternostomy in head injury: More questions than answers. Neurol India. 2022. 70: 1384
10. Parthiban JK, Sundaramahalingam S, Rao JB, Nannaware VP, Rathwa VN, Nasre VY. Basal cisternostomy-a microsurgical cerebro spinal fluid let out procedure and treatment option in the management of traumatic brain injury. Analysis of 40 consecutive head injury patients operated with and without bone flap replacement following cisternostomy in a tertiary care center in India. Neurol India. 2021. 69: 328
11. Peeters W, van den Brande R, Polinder S, Brazinova A, Steyerberg EW, Lingsma HF. Epidemiology of traumatic brain injury in Europe. Acta Neurochir. 2015. 157: 1683-96
12. Smith M, Citerio G. What’s new in subarachnoid hemorrhage. Intensive Care Med. 2015. 41: 123-6
13. Thapa A, Adhikari RB, Bidur KC, Shakya B. Role of cisternal drainage in patients with traumatic brain injury undergoing decompressive craniectomy. Nepal J Neurosci. 2018. 15: 14-20