- Departments of Neurosurgery, All India Institute of Medical sciences, CNC, AIIMS, New Delhi, India.
- Departments of Pathology, All India Institute of Medical sciences, CNC, AIIMS, New Delhi, India.
- Departments of Radio diagnosis, All India Institute of Medical sciences, CNC, AIIMS, New Delhi, India.
Correspondence Address:
Hitesh Gurjar
Departments of Radio diagnosis, All India Institute of Medical sciences, CNC, AIIMS, New Delhi, India.
DOI:10.25259/SNI_336_2019
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ravi Sharma, Varidh Katiyar, Hitesh Gurjar, Mehar Sharma, Revanth Goda, Zainab Vora. Is medulloblastoma associated with systemic immunomodulation? – A comparative analysis of preoperative inflammatory markers. 02-May-2020;11:86
How to cite this URL: Ravi Sharma, Varidh Katiyar, Hitesh Gurjar, Mehar Sharma, Revanth Goda, Zainab Vora. Is medulloblastoma associated with systemic immunomodulation? – A comparative analysis of preoperative inflammatory markers. 02-May-2020;11:86. Available from: https://surgicalneurologyint.com/surgicalint-articles/9994/
Abstract
Background: We attempt to compare preoperative inflammatory markers among children with medulloblastoma and pilocytic astrocytoma and establish their diagnostic efficacy to distinguish these tumors.
Methods: Children (
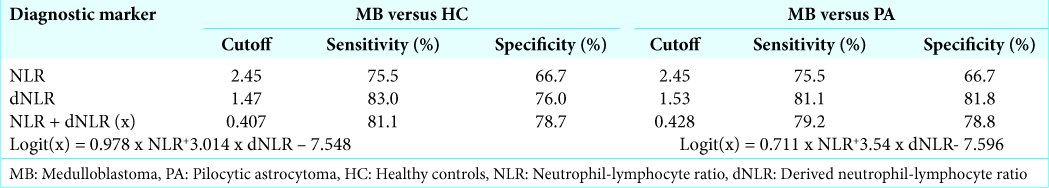
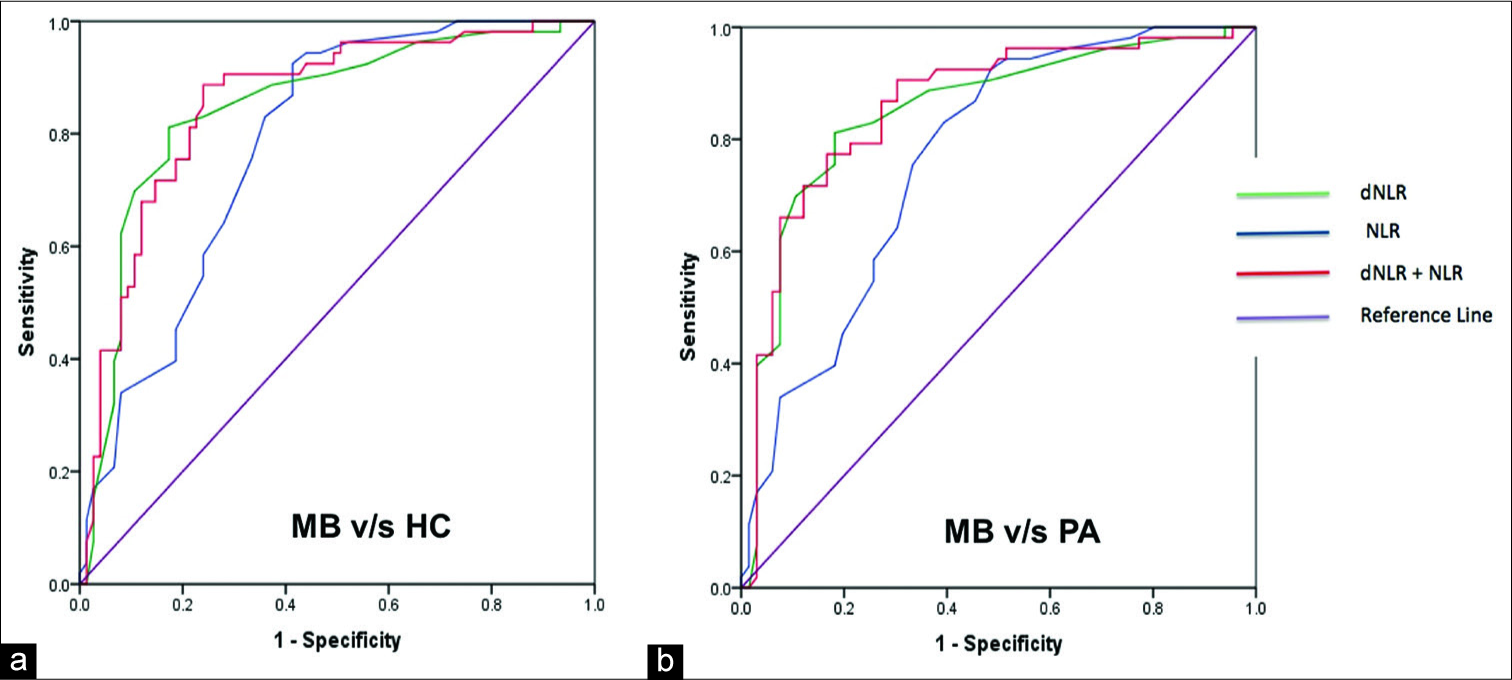
Results: Patients with medulloblastoma were found to have higher neutrophil-lymphocyte ratio (NLR), derived neutrophil-lymphocyte ratio (dNLR), platelet-lymphocyte ratio (PLR), and platelet counts compared with pilocytic astrocytoma. Absolute lymphocyte count (ALC) was significantly lower in medulloblastoma group as compared to healthy controls but not with pilocytic astrocytoma. NLR and dNLR demonstrated maximum diagnostic accuracy in distinguishing patients with medulloblastoma from healthy controls and pilocytic astrocytoma. Using a cutoff of 2.45 for NLR distinguishes medulloblastoma from healthy controls as well as pilocytic astrocytoma with a sensitivity of 75.5% and specificity of 66.7%. Similarly, dNLR cutoff of 1.47 distinguishes medulloblastoma from healthy controls with a sensitivity of 83% and specificity of 76% and a cutoff of 1.53 distinguishes medulloblastoma from pilocytic astrocytoma with a sensitivity of 81.1% and specificity of 81.8%. Combination of NLR and dNLR performed only marginally better than individual variables with area under the curve being 0.856 for medulloblastoma versus healthy controls and 0.86 for medulloblastoma versus pilocytic astrocytoma.
Conclusion: NLR and dNLR can be used as a preoperative predictive marker in medulloblastoma. There is decreased ALC in patients with medulloblastoma contributing to raised NLR and dNLR suggestive of systemic immunosuppression.
Keywords: Immunosuppression, Lymphopenia, Neutrophil-lymphocyte ratio
INTRODUCTION
Adult brain tumors have been found to be associated with systemic modulation of the immune system.[
Although the pediatric population is different in terms of the common types and location of brain tumors, few recent studies have shown that the immunological parameters are altered in the pediatric brain tumors as well.[
On this background, we attempt to compare these inflammatory markers among children with medulloblastoma (MB), pilocytic astrocytoma (PA), and healthy controls (HCs). We also aim to assess the efficacy of various inflammatory parameters and their combinations for differentiating medulloblastoma from pilocytic astrocytoma and healthy controls.
MATERIALS AND METHODS
Study design
The electronic medical records of pediatric population (<18 years) with biopsy-proven posterior fossa medulloblastoma and pilocytic astrocytoma operated at the Department of Neurosurgery, All India Institute of Medical Sciences, New Delhi, India, from January 2012 to January 2018 were collected and retrospectively analyzed. Patients who had recurrent tumor, had received preoperative chemotherapy and radiotherapy, preoperative hormonal replacement including glucocorticoids, history of infection or fever at the time of admission, history of autoimmune or hematological disease at the time of admission, history of serious hepatic, renal or cardiac dysfunction at the time of admission, history of medications which can alter blood parameters, or whose complete data on preoperative blood counts and serum albumin levels were not available were excluded from the study. Informed written consent was obtained from the guardian/parents of the child. For the control group, we included children who came to annual health check-up at the periphery screening centers of our institute with noninfectious/nonmalignant conditions such as lipomas and scoliosis,. This study was approved by the Institutional Ethics Committee.
Data collection
Information regarding the demographic and disease characteristics, including age, sex, and histopathology diagnosis, were retrieved from the hospital medical records and electronic database. Within 1 week before surgery, routine preoperative blood investigations were done. The TLC, NC, ALC, and PC were collected from the routine blood test, and albumin levels were collected from the hepatic function test. Other composite variables such as preoperative NLR (quotient of NC to LC), dNLR (quotient of NC to [TLC–NC]), PLR (ratio of PC to LC), and PNI (serum albumin (g/L)) + 5 × total lymphocyte count) were calculated as described.
Statistical analysis
Statistical analysis was performed using the SPSS version 20. Initially, normality of the distribution of the variables was analyzed using the Kolmogorov–Smirnov test. Since almost all variables deviated significantly from normality, nonparametric statistics were used for estimates of central tendency and hypothesis testing. Continuous variables were presented as median with interquartile range (IQR). For comparison between groups, the Mann–Whitney U-test was used. NLR, dNLR, PLR, and PNI of MB group were compared with pilocytic astrocytoma and healthy controls. The diagnostic performance of NLR, dNLR, PLR, PNI, and their combinations was assessed by values of the area under the curve (AUC) obtained from the receiver operating characteristic (ROC) curve. Cutoff points were estimated using ROC curves for the variables which demonstrated significant diagnostic accuracy using the maximum Youden index as the criteria. Throughout the analysis, a two-tailed P < 0.05 was considered statistically significant.
RESULTS
Study population
A total of 53 patients with medulloblastoma, 66 with pilocytic astrocytoma located in the posterior fossa and a control group of 75 healthy children were included in the final analysis. The demographic characteristics for the study subjects are presented in
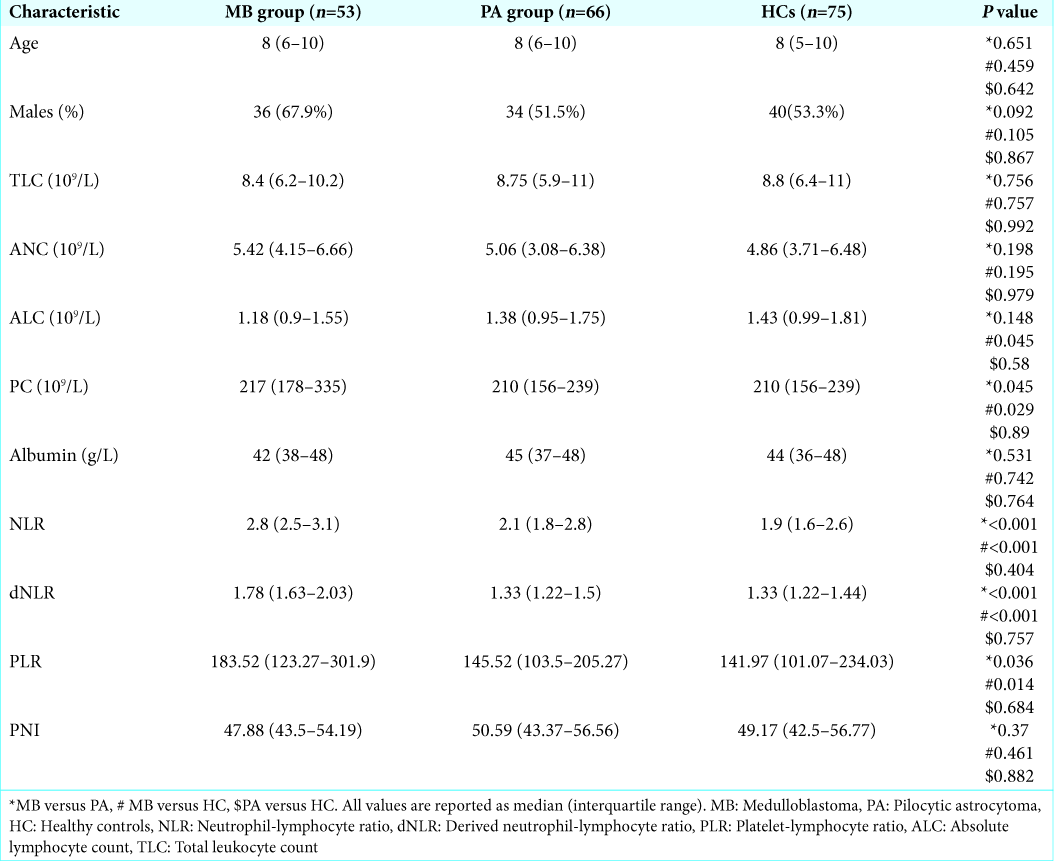
Comparison of preoperative inflammatory markers in medulloblastoma, pilocytic astrocytoma, and healthy controls
The preoperative inflammatory markers and comparisons between medulloblastoma, pilocytic astrocytoma, and healthy controls are detailed in
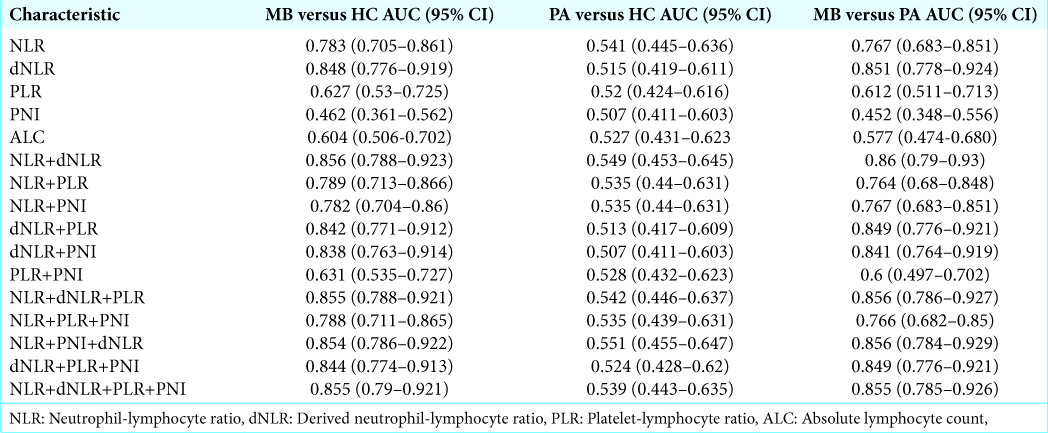
Evaluation of diagnostic efficacy of preoperative inflammatory markers and their combinations in patients with medulloblastoma versus healthy controls
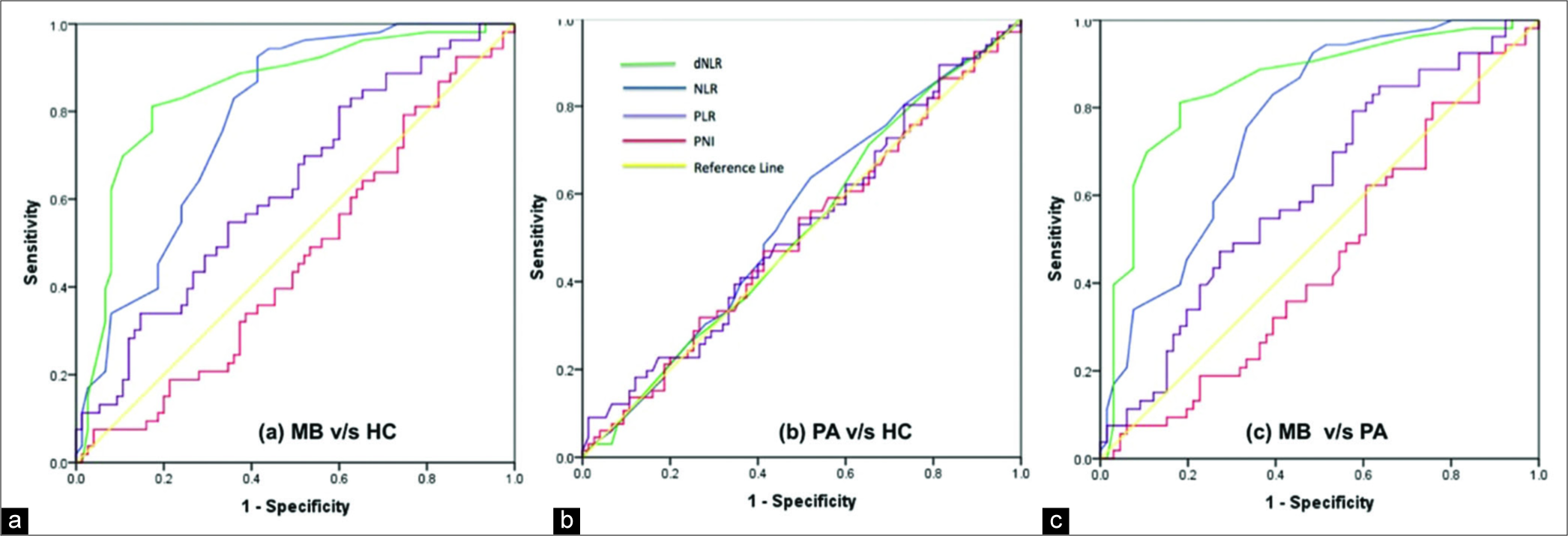
For individual parameters, NLR and dNLR demonstrated maximum diagnostic accuracy in distinguishing patients with medulloblastoma from healthy controls, with AUC of ROC curve analysis of 0.783 and 0.848, respectively [
Statistical prediction model for medulloblastoma
The performance of different combinations of all the inflammatory parameters as a diagnostic marker for distinguishing among medulloblastoma, pilocytic astrocytoma, and healthy controls group was assessed by constructing logistic regression model followed by their ROC curve analysis. Only the combination of NLR and dNLR had substantial value over individual variables to predict medulloblastoma in comparison to pilocytic astrocytoma and healthy controls, as discussed previously. The equations describing the logistic regression model to combine NLR and dNLR for medulloblastoma versus pilocytic astrocytoma and medulloblastoma versus healthy controls group are given in
DISCUSSION
There is recent surge of interest in the relationship between inflammation and tumor development and progression.[
In the light of these findings, several researchers have investigated the role of these blood markers in pediatric brain tumors as well.[
However, in another recent study conducted by Patel et al.,[
Wilson et al.[
We analyzed the diagnostic efficiency of the various markers using ROC curves and found NLR and dNLR to be the only significant factor that can accurately predict medulloblastoma vis-à-vis pilocytic astrocytoma or healthy controls. ALC had considerably less accuracy with maximum Youden index of only 0.236 to distinguish medulloblastoma from healthy controls, though it was found to be significantly different. We observed that the correlation of NLR and dNLR could primarily be ascribed to neutrophilia rather than lymphopenia in our sample. NLR cutoffs of 2.45 and 2.45 and dNLR cutoffs of 1.47 and 1.53 had a good predictive accuracy for medulloblastoma when compared to pilocytic astrocytoma or healthy controls, respectively, in the pediatric population. Out of the combined markers, only NLR and dNLR combination was found to have significant predictive value. Although no other hematological marker was found to be of value in our study, their relevance cannot be ruled out based on the existing literature. Although Patel et al.[
This study suffers from a few limitations. The most important drawback of the study being the symptomatology of these tumors commonly being vomiting and the treatment patient received for vomiting which possibly can affect immunological status of the patient, but we tried to exclude the patients who had a history of infection or who had received antibiotics before admission. The other drawbacks of the study are that the sample size of the study was small and this might have been the reason that a few markers failed to show significant correlation. This was a unicentric retrospective case–control study, in which we attempted to eliminate the possible sources of bias. However, large prospective studies with stringent selection criteria to eliminate bias are needed to verify the findings and provide a more accurate estimation of the diagnostic accuracy and cutoffs of these parameters. Considering the difference in the behavior of various molecular subtypes of medulloblastoma, it would be interesting to note the efficacy of these parameters in differentiating these subtypes. We did not include this analysis as this information was not available for some of our patients and the remaining number was too small to be of any statistical value. We have also not analyzed the long-term outcomes of the patients and thus the possible role of the parameters as a prognostic marker due to lack of follow-up data of the patients.
Apart from these limitations, we would like to discuss the clinical relevance of our study. Although the role of these biomarkers has been extensively studied in the adult glioblastoma patients, there is not much literature on the most common pediatric high-grade tumor, medulloblastoma. There is a study evaluating all the pediatric intracranial SOLs and evaluating the role of these parameters in predicting grade of these tumors irrespective of the histological type of tumor.[
CONCLUSION
The current evidence strongly suggests the interrelationship between immunological alterations and the pathogenesis and progression of medulloblastoma. It seems that NLR and dNLR can complement the imaging features in diagnosis of medulloblastomas in children similar to GBM in adults. However, the quantitative details of these findings and the underlying pathophysiologic mechanisms have not been clearly elucidated. Our study gives an important directive for future research in the involvement of immune system and prognostic value of these factors in medulloblastoma patients.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Baecher-Allan C, Anderson DE. Regulatory cells and human cancer. Semin Cancer Biol. 2006. 16: 98-105
2. Bambury RM, Teo MY, Power DG, Yusuf A, Murray S, Battley JE. The association of pre-treatment neutrophil to lymphocyte ratio with overall survival in patients with glioblastoma multiforme. J Neurooncol. 2013. 114: 149-54
3. Bhatti I, Peacock O, Lloyd G, Larvin M, Hall RI. Preoperative hematologic markers as independent predictors of prognosis in resected pancreatic ductal adenocarcinoma: Neutrophil-lymphocyte versus platelet-lymphocyte ratio. Am J Surg. 2010. 200: 197-203
4. Dagistan Y, Dagistan E, Citisli V. Evaluation of simple blood counts as inflammation markers for brain tumor patients. Neurol Neurochir Pol. 2016. 50: 231-5
5. Das S, Marsden PA. Angiogenesis in glioblastoma. N Engl J Med. 2013. 369: 1561-3
6. Fossati G, Ricevuti G, Edwards SW, Walker C, Dalton A, Rossi ML. Neutrophil infiltration into human gliomas. Acta Neuropathol. 1999. 98: 349-54
7. Garcea G, Ladwa N, Neal CP, Metcalfe MS, Dennison AR, Berry DP. Preoperative neutrophil-to-lymphocyte ratio (NLR) is associated with reduced disease-free survival following curative resection of pancreatic adenocarcinoma. World J Surg. 2011. 35: 868-72
8. Gomez D, Farid S, Malik HZ, Young AL, Toogood GJ, Lodge JP. Preoperative neutrophil-to-lymphocyte ratio as a prognostic predictor after curative resection for hepatocellular carcinoma. World J Surg. 2008. 32: 1757-62
9. Gustafson MP, Lin Y, New KC, Bulur PA, O’Neill BP, Gastineau DA. Systemic immune suppression in glioblastoma: The interplay between CD14+HLA-DRlo/neg monocytes, tumor factors, and dexamethasone. Neuro Oncol. 2010. 12: 631-44
10. Han S, Zhang C, Li Q, Dong J, Liu Y, Huang Y. Tumour-infiltrating CD4(+) and CD8(+) lymphocytes as predictors of clinical outcome in glioma. Br J Cancer. 2014. 110: 2560-8
11. Jia J, Zheng X, Chen Y, Wang L, Lin L, Ye X. Stage-dependent changes of preoperative neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in colorectal cancer. Tumour Biol. 2015. 36: 9319-25
12. Jung MR, Park YK, Jeong O, Seon JW, Ryu SY, Kim DY. Elevated preoperative neutrophil to lymphocyte ratio predicts poor survival following resection in late stage gastric cancer. J Surg Oncol. 2011. 104: 504-10
13. Kang MH, Go SI, Song HN, Lee A, Kim SH, Kang JH. The prognostic impact of the neutrophil-to-lymphocyte ratio in patients with small-cell lung cancer. Br J Cancer. 2014. 111: 452-60
14. Kawata A, Une Y, Hosokawa M, Uchino J, Kobayashi H. Tumor-infiltrating lymphocytes and prognosis of hepatocellular carcinoma. Jpn J Clin Oncol. 1992. 22: 256-63
15. Kilincalp S, Çoban Ş, Akinci H, Hamamcı M, Karaahmet F, Coşkun Y. Neutrophil/lymphocyte ratio, platelet/ lymphocyte ratio, and mean platelet volume as potential biomarkers for early detection and monitoring of colorectal adenocarcinoma. Eur J Cancer Prev. 2015. 24: 328-33
16. Kishi Y, Kopetz S, Chun YS, Palavecino M, Abdalla EK, Vauthey JN. Blood neutrophil-to-lymphocyte ratio predicts survival in patients with colorectal liver metastases treated with systemic chemotherapy. Ann Surg Oncol. 2009. 16: 614-22
17. McNamara MG, Lwin Z, Jiang H, Templeton AJ, Zadeh G, Bernstein M. Factors impacting survival following second surgery in patients with glioblastoma in the temozolomide treatment era, incorporating neutrophil/lymphocyte ratio and time to first progression. J Neurooncol. 2014. 117: 147-52
18. Patel S, Wang S, Snuderl M, Karajannis MA. Pre-treatment lymphopenia and indication of tumor-induced systemic immunosuppression in medulloblastoma. J Neurooncol. 2018. 136: 541-4
19. Rutledge WC, Kong J, Gao J, Gutman DA, Cooper LAD, Appin C. Tumor-infiltrating lymphocytes in glioblastoma are associated with specific genomic alterations and related to transcriptional class. Clin Cancer Res. 2013. 19: 4951-60
20. Sowers JL, Johnson KM, Conrad C, Patterson JT, Sowers LC. The role of inflammation in brain cancer. Adv Exp Med Biol. 2014. 816: 75-105
21. Tumturk A, Ozdemir MA, Per H, Unal E, Kucuk A, Ulutabanca H. Pediatric central nervous system tumors in the first 3 years of life: Pre-operative mean platelet volume, neutrophil/lymphocyte count ratio, and white blood cell count correlate with the presence of a central nervous system tumor. Childs Nerv Syst. 2017. 33: 233-8
22. Wilson JR, Saeed F, Tyagi AK, Goodden JR, Sivakumar G, Crimmins D. Pre-operative neutrophil count and neutrophil-lymphocyte count ratio (NLCR) in predicting the histological grade of paediatric brain tumours: A preliminary study. Acta Neurochir (Wien). 2018. 160: 793-800
23. Zheng S, Huang J, Chen M, Wang B, Ou Q, Huang S. Diagnostic value of preoperative inflammatory markers in patients with glioma: A multicenter cohort study. J Neurosurg. 2018. 129: 583-92