- Department of Neurosurgery, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India.
- Department of Radiodiagnosis, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India.
- Department of Anaesthesiology and Critical Care, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India.
Correspondence Address:
Ramesh Andi Sadayandi, Department of Neurosurgery, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India.
DOI:10.25259/SNI_318_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Dinesh Verma1, Ramesh Andi Sadayandi1, Sathiaprabhu Anbazhagan1, Krishnan Nagarajan2, Prasanna Udupi Bidkar3. Is optic nerve sheath diameter a promising screening tool to predict neurological outcomes and the need for secondary decompressive craniectomy in moderate to severe head injury patients? A prospective monocentric observational pilot study. 04-Aug-2023;14:276
How to cite this URL: Dinesh Verma1, Ramesh Andi Sadayandi1, Sathiaprabhu Anbazhagan1, Krishnan Nagarajan2, Prasanna Udupi Bidkar3. Is optic nerve sheath diameter a promising screening tool to predict neurological outcomes and the need for secondary decompressive craniectomy in moderate to severe head injury patients? A prospective monocentric observational pilot study. 04-Aug-2023;14:276. Available from: https://surgicalneurologyint.com/surgicalint-articles/12484/
Abstract
Background: Optic nerve sheath diameter (ONSD) has been shown to be a noninvasive and quick method to calculate intracranial pressure (ICP) and subsequent neurologic outcomes, although with variable cutoffs. ICP can be indirectly assessed by noninvasive methods such as transcranial Doppler, ONSD, tympanic membrane displacement, and fundoscopy. Knowledge regarding the diagnostic accuracy of ONSD for predicting unfavorable outcomes within 72 hours (h) of moderate and severe head injury is limited. The objective of this study was to measure ONSD measurements at 24-h intervals in moderate to severe head injury patients and to find its association with clinical outcomes in the target population.
Methods: This prospective observational study was done on moderate to severe head injury patients. ONSD was measured twice at 24-h intervals over 48 h. The clinical outcome was divided into the favorable group (patients who were in conservative treatment with a stable Glasgow Coma Scale [GCS] score and discharged following treatment) and the unfavorable group (patients who had a drop in GCS motor score of one or more, or expired or underwent surgical intervention) within 72 h following traumatic brain injury. The Kruskal–Wallis test, Mann– Whitney test, and receiver operating characteristic curves were used to establish the association between ONSD and clinical outcomes.
Results: ONSD values measured at 24-h intervals >6.1 mm (P P
Conclusion: ONSD is an efficient screening tool to assess neurological outcomes in severe head injury patients. It can reliably predict the need for secondary DC at an earlier stage before secondary brain damage ensues in these patients.
Keywords: Decompressive craniectomy, Neurological outcome, Optic nerve sheath diameter, Traumatic brain injury
INTRODUCTION
Intracranial pressure (ICP) rises in various neurological and nonneurological conditions and requires rapid diagnosis and treatment due to its fulminant nature.[
MATERIALS AND METHODS
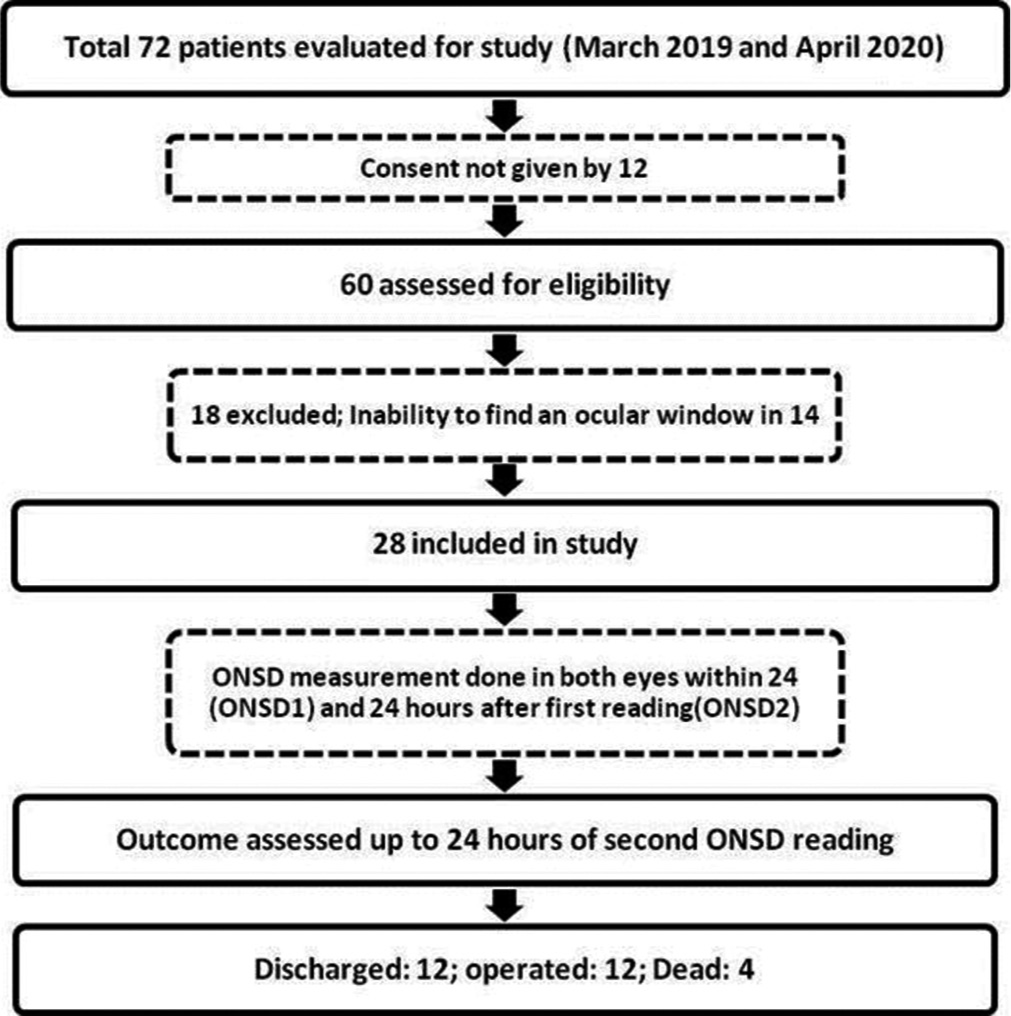
This study was conducted as a single-center prospective observational study at a tertiary care referral center for neurological disorders in South India between March 2019 and April 2020. The study was prior reviewed and approved by the Postgraduate Research Monitoring Committee and the Institute Ethics Committee [JIP/IEC/2018/166].
Inclusion criteria
Individuals between 18 and 60 who were admitted to the hospital ED within 24 h following moderate and severe head injuries (Glasgow coma scale [GCS] score 3–12) were included in the study.
Exclusion criteria
Patients with active medical conditions such as diabetes mellitus, hypertension, ophthalmic disorders with optic nerve changes, polytrauma, hemodynamically unstable, penetrating head injury, skull base fracture with cerebrospinal fluid (CSF) leak, and ocular lesions precluding assessment of ONSD were excluded from the study.
Sampling method
Based on a pilot study by Robba et al., the area under the curve (AUC) of ICP in predicting surgical intervention was 0.75.[
Measurement of ONSD
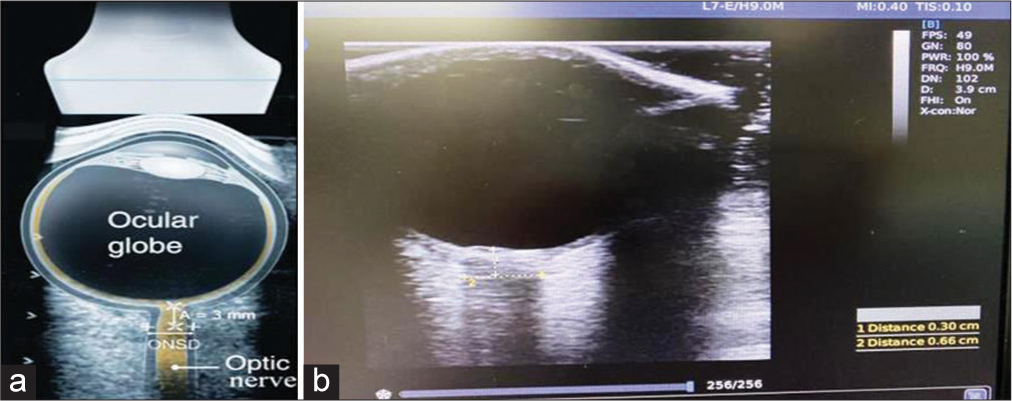
Patients who were planned for conservative management on admission based on the clinical examination, computerized tomography (CT) brain findings, and BTF guidelines were taken for ONSD measurements over a period of 48 h. Three readings were taken from each eye by a single investigator (resident neurosurgeon) twice with an interval of 24 h. The mean ONSD value was calculated from the average of six readings each time. The first mean ONSD reading was taken in EMS or trauma intensive care unit (ICU) within 24 h of TBI (ONSD1), and the second was taken after 24 h (ONSD2) from the first reading in the trauma ICU. If the interval between the first CT brain and ONSD1 measurement happened to be more than 6 h, a repeat CT was taken before the ONSD measurement to ensure the patient management (conservative as per initial assessment) remained unaffected. A total of 336 ONSD measurements were taken from 28 moderate to severe head injury patients (3 in each eye 24 h apart). The average ONSD measurement in each eye was utilized in statistical analysis to minimize the effects of the laterality of any lesion. Fifty-six average ONSD measurements were obtained in the study.
Since most TBI patients tend to deteriorate in the first 72 h of TBI, all the participants were observed until 24 h following the second ONSD measurement (ONSD2).[
The ONSD was measured using a 7.5-MHz linear ultrasound probe (CHISON digital color Doppler system Ebit60) with the lowest possible acoustic power that could measure the ONSD [
Clinical outcome
The clinical outcome of importance was defined as a drop in GCS by a motor score of one or more, or surgical intervention, or death within 24 h following ONSD2 measurements. The clinical outcomes were compared with ONSD values and divided into the favorable group (patients who were in conservative treatment with a stable GCS score and discharged following treatment) and unfavorable group (patients who had a drop in GCS motor score of one or more, or expired or underwent surgical intervention) within 72 h following TBI.[
Statistical method
The data were entered in the MS EXCEL spreadsheet, and analysis was done using the Statistical Package for the Social Sciences version 21.0, IBM, USA. Continuous variables were reported as mean ± standard deviation and median, whereas categorical variables were presented as numbers and percentages. The receiver operating characteristic (ROC) curve was used to find out the AUC of ONSD for predicting surgery. Kruskal–Wallis test was applied to find the association between ONSD with the outcome. Mann–Whitney test was applied to establish the relation between ONSD and clinical outcome. The ROC curve of ONSD was plotted to predict an unfavorable outcome. P < 0.05 was considered statistically significant. The sensitivity, specificity, and DA of ONSD were assessed.
RESULTS
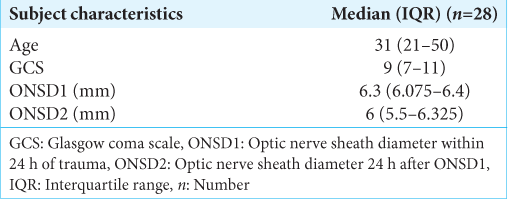
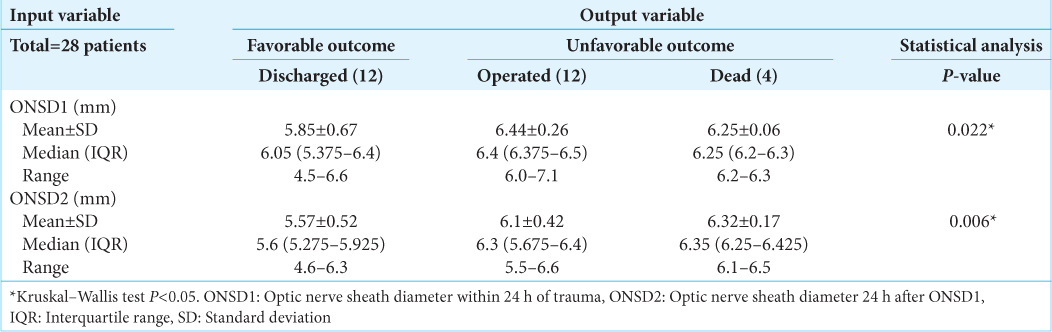
Twenty-eight patients (21 males and seven females) with moderate and severe head injuries were enrolled in this study. The median (interquartile range) age, GCS score, ONSD1, and ONSD2 of the study participants were 31 (21–50), 9 (7–11), 6.3 mm (6.075–6.4), and 6 mm (5.5–6.325), respectively [
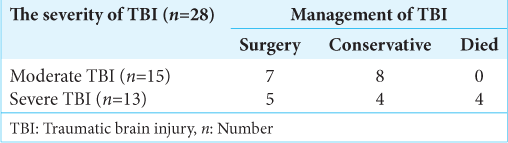
Among the 28 study subjects, 12 patients (43%) had no deterioration in GCS score during conservative management and were subsequently discharged. Twelve patients (43%) underwent DC, and 4 (14%) expired [
ONSD and clinical outcomes
Significantly different ONSD1 values were observed between the discharged (6.05 mm), dead (6.25 mm), and operated (6.4 mm) groups (P = 0.022). Similarly, significantly different ONSD2 values were observed between the discharged (5.6 mm), dead (6.35 mm), and operated (6.3 mm) groups (P = 0.006) [
DA of ONSD1 and 2
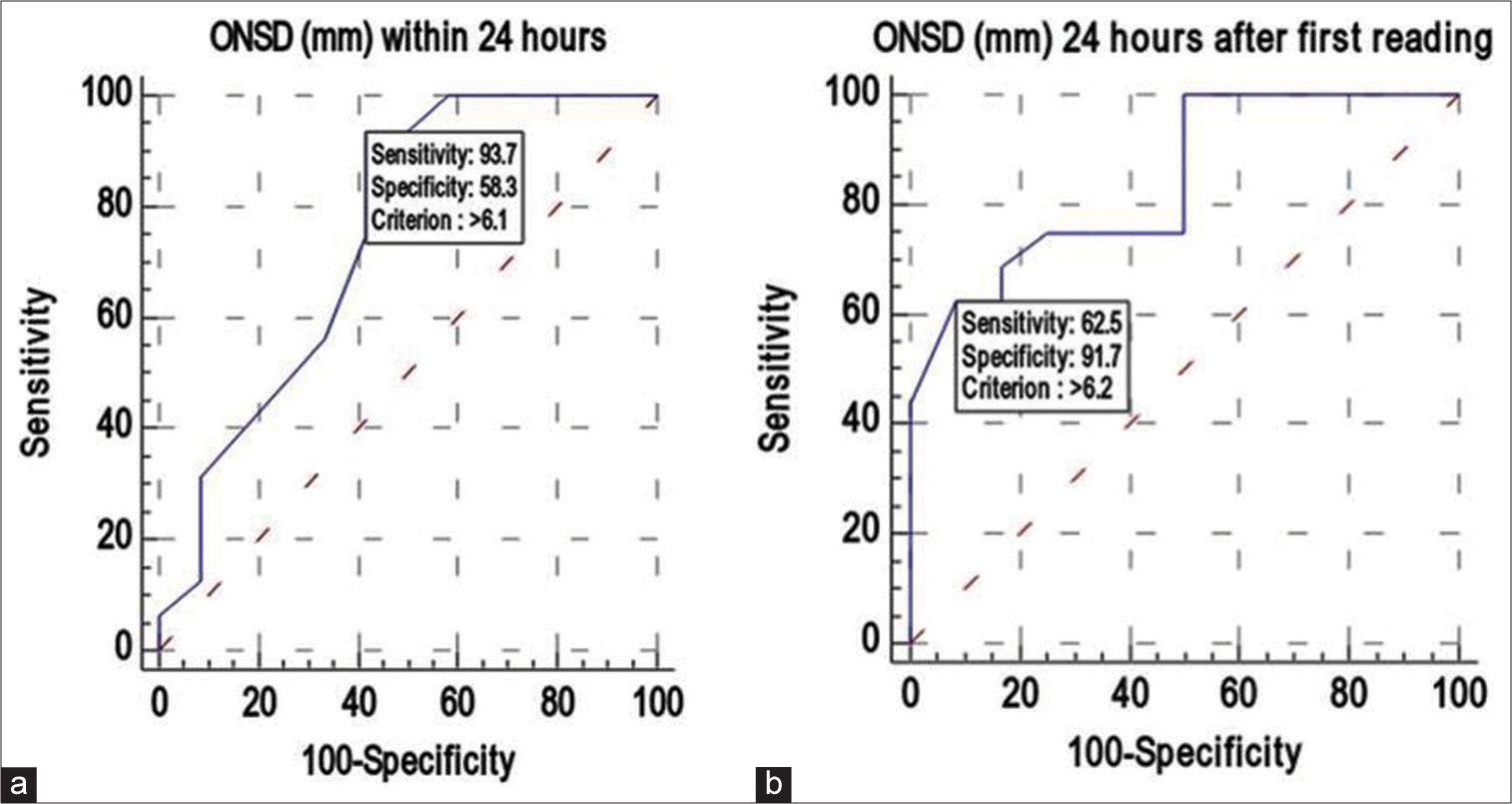
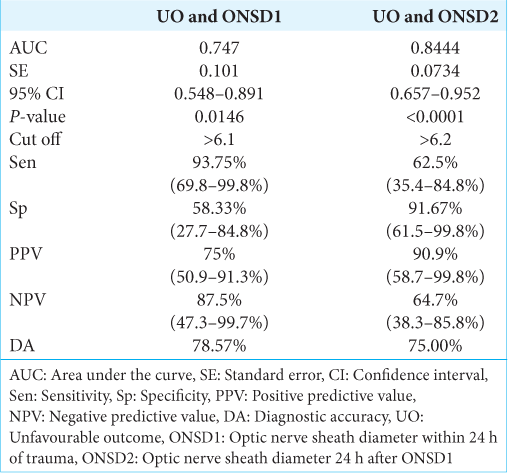
ONSD1 had a strong discriminating power (AUC 0.747; 95% confidence interval (CI): 0.548–0.891), and an ONSD1 value >6.1 mm was found in 93.75% (sensitivity) of patients with unfavorable outcomes. If the ONSD1 valve was >6.1 mm, there was a 75% positive predictive value (PPV) of predicting an unfavorable outcome. An ONSD1 value was ≤6.1 mm indicated a favorable outcome. The DA of ONSD1 (mm) in predicting unfavorable outcomes was 78.57% [
Similarly, ONSD2 also had a strong discriminating power (AUC 0.844; 95% CI: 0.657–0.952), and ONSD2 value >6.2 mm was found in 62.5% (sensitivity) of patients with unfavorable outcomes. If the ONSD2 valve was >6.2 mm, there was a 90.9% PPV of predicting an unfavorable outcome. An ONSD2 value was ≤6.2 mm indicated a favorable outcome. The DA of ONSD2 (mm) in predicting unfavorable outcomes was 75% [
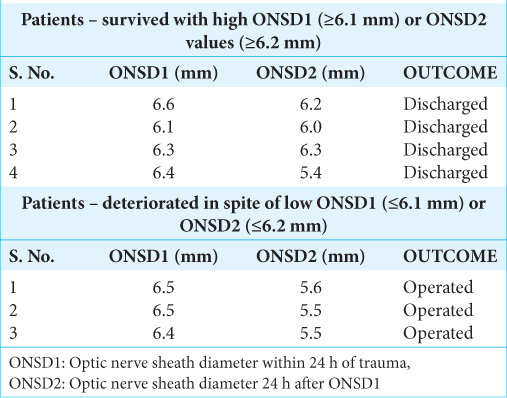
It was observed that four patients, despite high ONSD values, survived, while three patients with low ONSD values deteriorated and underwent surgery [
DISCUSSION
The primary aim of this study was to measure the ONSD among 28 patients with moderate to severe head injury and to determine the relationship between ONSD and clinical outcomes in these patients. Significantly different ONSD1 and ONSD2 values were observed between the discharged, dead, and operated groups. The DA of ONSD1 and ONSD2 in predicting unfavorable outcomes was 78.57% and 75%.
The novelty of this study is that it has demonstrated the reliability of ONSD to predict neurological outcomes and the need for secondary DC in the early stages of TBI, where clinical signs and computerized tomography (CT) brain findings are of ambiguous nature. To the best of our knowledge, this is the first study where ONSD values were taken in both eyes at regular intervals in all the study participants, twice within 72 h of TBI, during which most of the patients tend to deteriorate due to intracranial hypertension and loss of autoregulation.[
The need for noninvasive methods in TBI patients
Invasive ICP monitoring is presently considered the standard of care in moderate and severe head injury patients despite its lack of level I evidence. Physicians often have to make decisions on a case-by-case basis in the management, particularly in borderline scenarios.[
The role of ONSD in TBI patients monitoring
Techniques such as TCD, ONSD, tympanic membrane (TM) displacement, fundoscopy, and CT/magnetic resonance imaging (MRI) may be helpful in assessing the ICP by noninvasive methods. Among these, ONSD can be used as a potential screening tool for TBI patients because it is quick, efficient, and can differentiate between normal and raised ICP at the bedside. TCD has a high percentage of unsuccessful measurements. Fundoscopy and MRI have a limited role in TBI.[
The advantages of ONSD measurement by USG over CT or MRI
ONSD was measured by USG in previous studies such as Robba et al., Strumwasser et al., Geeraerts et al., Thotakura et al., Rajajee et al., Wang et al., and Soldatos et al.[
Association between ONSD and clinical outcomes
ONSD1 value >6.1 mm was found in 93.75% of patients with unfavorable outcomes. It was also observed that if the ONSD2 valve was >6.2 mm, then there was a 90.9% probability of predicting unfavorable outcomes. The study could predict that if the ONSD value was <6.1 mm in the study participants, the chance of a favorable outcome was up to 87.5% [
Variation in cutoff values of ONSD
The optic nerve sheath is anatomically continuous with the dura mater of the brain, and it has a trabeculated subarachnoid space through which CSF flows. The fibrillary arrangement of arachnoid trabeculations allows the optic nerve sheath to dilate when ICP rises in TBI. The trabeculations in the anterior segment of the optic nerve sheath are sparse with respect to the posterior segment making it more distensible than the posterior segment.[
Studies by Geeraerts et al., and Kimberly and Noble, have shown that the cutoff value of ONSD to predict the raised ICP was between 4.5 and 6.3 mm. Variations in ONSD have been attributed to multiple reasons, including the technique used, variations in the machine and probe, the experience of the observer, and the study population from various ethnicities.[
DA of ONSD in predicting unfavorable outcomes
Studies by Robba et al., Rajajee et al., and Wang et al. have proved the high sensitivity and specificity value of ONSD in their analysis, but only a few studies have discussed its DA.[
ONSD as a screening tool
Thus, the study has clearly demonstrated that ONSD could be used as a potential screening tool to infer ICP indirectly (noninvasive ICP) in moderate and severe head injury patients, the reasons being its measurement by USG, which is less time-consuming, accurate, efficient, low-cost, noninvasive, and can differentiate normal and raised ICP. While USG is readily available in many peripheral centers, the facility to measure the invasive ICP is not available or contraindicated due to various clinical conditions. This study has also proved that ONSD can predict favorable and unfavorable clinical outcomes and the need for DC in the early stages of TBI by a noninvasive method.
The limitations of the techniques, like intra/interobserver variability and nonavailability of the optimal orbital window due to ocular disorders, preclude its use as a definite substitute for invasive methods.
Limitations
The major limitations of this study are its small sample size, and short follow-up period (up to 72 h following head injury). Considering the age group of the study participants, the study results cannot be generalized to the entire population. Hence, studies with a larger sample size involving individuals of different age groups are required to substantiate the results of the study.
CONCLUSION
The ONSD is a potential screening tool for predicting favorable and unfavorable clinical outcomes in moderate and severe head injury patients. It will also provide valuable information about the need for early DC, which will avoid potential loss of time and help the patient to gain a favorable neurological outcome.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Al-Jishi A, Saluja RS, Al-Jehani H, Lamoureux J, Maleki M, Marcoux J. Primary or secondary decompressive craniectomy: Different indication and outcome. Can J Neurol Sci. 2011. 38: 612-20
2. Bekerman I, Sigal T, Kimiagar I, Ely AB, Vaiman M. The quantitative evaluation of intracranial pressure by optic nerve sheath diameter/eye diameter CT measurement. Am J Emerg Med. 2016. 34: 2336-42
3. Bratton SL. Guidelines for the management of severe traumatic brain injury. IX. Cerebral perfusion thresholds. J Neurotrauma. 2007. 24: S59-64
4. Carney N, Totten AM, O’Reilly C, Ullman JS, Hawryluk GWJ, Bell MJ. Guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery. 2017. 80: 6-15
5. Chesnut RM, Temkin N, Carney N, Dikmen S, Rondina C, Videtta W. A trial of intracranial-pressure monitoring in traumatic brain injury. N Engl J Med. 2012. 367: 2471-81
6. Cnossen MC, Huijben JA, van der Jagt M, Volovici V, van Essen T, Polinder S. Variation in monitoring and treatment policies for intracranial hypertension in traumatic brain injury: A survey in 66 neurotrauma centers participating in the CENTER-TBI study. Crit Care Lond Engl. 2017. 21: 233
7. De Riva N, Budohoski KP, Smielewski P, Kasprowicz M, Zweifel C, Steiner LA. Transcranial Doppler pulsatility index: What it is and what it isn’t. Neurocrit Care. 2012. 17: 58-66
8. Dubourg J, Javouhey E, Geeraerts T, Messerer M, Kassai B. Ultrasonography of optic nerve sheath diameter for detection of raised intracranial pressure: A systematic review and meta-analysis. Intensive Care Med. 2011. 37: 1059-68
9. Gao Y, Li Q, Wu C, Liu S, Zhang M. Diagnostic and prognostic value of the optic nerve sheath diameter with respect to the intracranial pressure and neurological outcome of patients following hemicraniectomy. BMC Neurol. 2018. 18: 199
10. Geeraerts T, Merceron S, Benhamou D, Vigué B, Duranteau J. Non-invasive assessment of intracranial pressure using ocular sonography in neurocritical care patients. Intensive Care Med. 2008. 34: 2062-7
11. Geeraerts T, Newcombe VF, Coles JP, Abate MG, Perkes IE, Hutchinson PJ. Use of T2-weighted magnetic resonance imaging of the optic nerve sheath to detect raised intracranial pressure. Crit Care Lond Engl. 2008. 12: R114
12. Hansen HC, Helmke K. The subarachnoid space surrounding the optic nerves. An ultrasound study of the optic nerve sheath. Surg Radiol Anat SRA. 1996. 18: 323-8
13. Hansen HC, Helmke K. Validation of the optic nerve sheath response to changing cerebrospinal fluid pressure: Ultrasound findings during intrathecal infusion tests. J Neurosurg. 1997. 87: 34-40
14. Helmke K, Hansen HC. Fundamentals of transorbital sonographic evaluation of optic nerve sheath expansion under intracranial hypertension. I. Experimental study. Pediatr Radiol. 1996. 26: 701-5
15. Kim YH, Lee JH, Hong CK, Cho KW, Yeo JH, Kang MJ. Feasibility of optic nerve sheath diameter measured on initial brain computed tomography as an early neurologic outcome predictor after cardiac arrest. Acad Emerg Med. 2014. 21: 1121-8
16. Kimberly HH, Noble VE. Using MRI of the optic nerve sheath to detect elevated intracranial pressure. Crit Care. 2008. 12: 181
17. Legrand A, Jeanjean P, Delanghe F, Peltier J, Lecat B, Dupont H. Estimation of optic nerve sheath diameter on an initial brain computed tomography scan can contribute prognostic information in traumatic brain injury patients. Crit Care Lond Engl. 2013. 17: R61
18. Raboel PH, Bartek J, Andresen M, Bellander BM, Romner B. Intracranial pressure monitoring: Invasive versus non-invasive methods-a review. Crit Care Res Pract. 2012. 2012: 950393
19. Rajajee V, Vanaman M, Fletcher JJ, Jacobs TL. Optic nerve ultrasound for the detection of raised intracranial pressure. Neurocrit Care. 2011. 15: 506-15
20. Robba C, Cardim D, Tajsic T, Pietersen J, Bulman M, Donnelly J. Ultrasound non-invasive measurement of intracranial pressure in neurointensive care: A prospective observational study. PLoS Med. 2017. 14: e1002356
21. Robba C, Donnelly J, Cardim D, Tajsic T, Cabeleira M, Citerio G. Optic nerve sheath diameter ultrasonography at admission as a predictor of intracranial hypertension in traumatic brain injured patients: A prospective observational study. J Neurosurg. 2019. 132: 1279-85
22. Sekhon MS, McBeth P, Zou J, Qiao L, Kolmodin L, Henderson WR. Association between optic nerve sheath diameter and mortality in patients with severe traumatic brain injury. Neurocrit Care. 2014. 21: 245-52
23. Soldatos T, Karakitsos D, Chatzimichail K, Papathanasiou M, Gouliamos A, Karabinis A. Optic nerve sonography in the diagnostic evaluation of adult brain injury. Crit Care Lond Engl. 2008. 12: R67
24. Strumwasser A, Kwan RO, Yeung L, Miraflor E, Ereso A, Castro-Moure F. Sonographic optic nerve sheath diameter as an estimate of intracranial pressure in adult trauma. J Surg Res. 2011. 170: 265-71
25. Tayal VS, Neulander M, Norton HJ, Foster T, Saunders T, Blaivas M. Emergency department sonographic measurement of optic nerve sheath diameter to detect findings of increased intracranial pressure in adult head injury patients. Ann Emerg Med. 2007. 49: 508-14
26. Thotakura AK, Marabathina NR, Danaboyina AR, Mareddy RR. Role of serial ultrasonic optic nerve sheath diameter monitoring in head injury. Neurochirurgie. 2017. 63: 444-8
27. Wang J, Li K, Li H, Ji C, Wu Z, Chen H. Ultrasonographic optic nerve sheath diameter correlation with ICP and accuracy as a tool for noninvasive surrogate ICP measurement in patients with decompressive craniotomy. J Neurosurg. 2020. 133: 514-520
28. Wang LJ, Chen LM, Chen Y, Bao LY, Zheng NN, Wang YZ. Ultrasonography assessments of optic nerve sheath diameter as a noninvasive and dynamic method of detecting changes in intracranial pressure. JAMA Ophthalmol. 2018. 136: 250-6
29. Zweifel C, Czosnyka M, Carrera E, de Riva N, Pickard JD, Smielewski P. Reliability of the blood flow velocity pulsatility index for assessment of intracranial and cerebral perfusion pressures in head-injured patients. Neurosurgery. 2012. 71: 853-61