- Department of Neurosurgery, Queen Elizabeth Hospital, Birmingham, United Kingdom.
- Institute of Translational Medicine, Queen Elizabeth Hospital, Birmingham, United Kingdom.
Correspondence Address:
Anne S. L. Elserius, Department of Neurosurgery, Queen Elizabeth University Hospital, Mindelsohn Way, Birmingham, United Kingdom.
DOI:10.25259/SNI_438_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Anne S. L. Elserius1, James Hodson2, Athanasios Zisakis1, Ismail Ughratdar1. Is there a limited value of cytoreductive surgery in elderly patients with malignant gliomas?. 22-Jul-2022;13:320
How to cite this URL: Anne S. L. Elserius1, James Hodson2, Athanasios Zisakis1, Ismail Ughratdar1. Is there a limited value of cytoreductive surgery in elderly patients with malignant gliomas?. 22-Jul-2022;13:320. Available from: https://surgicalneurologyint.com/surgicalint-articles/11730/
Abstract
Background: Glioblastoma (GB) is well known for being the most aggressive primary cerebral malignancy. The peak incidence is at 60–70 years of age, with over half of patients aged over 65 years at diagnosis.
Methods: Patients with a confirmed histological diagnosis of GB between January 2009 and June 2016 at a single center were retrospectively identified. The inclusion criteria for the study were age over 65 years at diagnosis, and surgical management with either a burr hole biopsy or craniotomy.
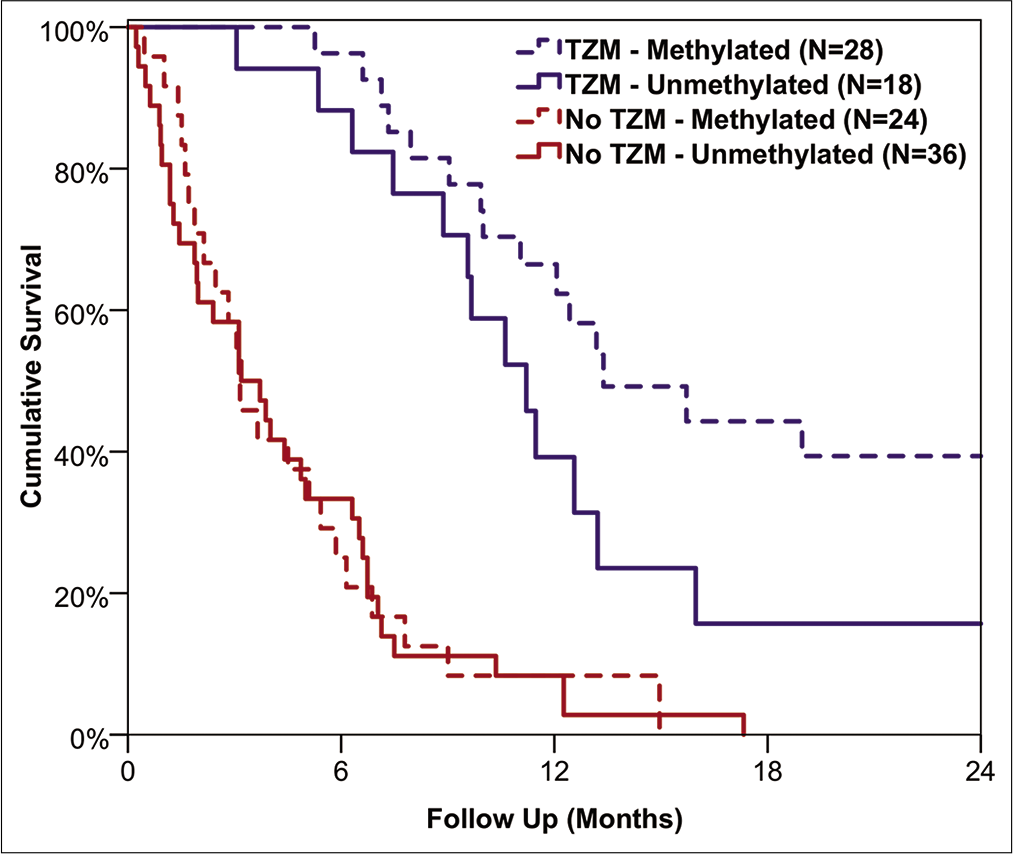
Results: A total of n = 289 patients underwent surgery for GB, with a median age at diagnosis of 71 years, and of whom 64% were male. Craniotomies were performed in 71%, with burr hole biopsies performed in the remainder (29%). Patient survival differed significantly with treatment modality (P P P = 0.006). For the subgroup of patients receiving TZM, those with a methylated O6-methylguanine-DNA-methyltransferase (MGMT) status had significantly longer overall survival than those with unmethylated MGMT (median: 407 vs. 341 days, P = 0.039).
Conclusion: Our retrospective data demonstrate that the elderly population with GB benefit from aggressive chemo-RT, regardless of surgical intervention.
Keywords: Chemotherapy, Craniotomy, Elderly, Glioblastoma, Radiotherapy
INTRODUCTION
With an increase of the worldwide population over the age of 65 from 8.5% (617 million) in 2016 to a projected 25.5% (1.6 billion) in 2050,[
There is consensus in the literature that several factors in addition to age play a role in outcomes and survival for elderly patients. Compared to younger patient groups, comorbidities and performance status (PS) have greater impact on the individual treatment decision in elderly patients,[
Since the publication of data by Perry et al.,[
MATERIALS AND METHODS
Patient population
Patients were identified from a local oncology database at our unit in Birmingham, UK. This database contains patients from a single catchment population in and around Birmingham, UK. The patients in this study were selected based on multidisciplinary decisions for surgical intervention, whether this was a simple biopsy for histopathological confirmation of GB to then facilitate an appropriate neuro-oncology management, or a more aggressive surgical debulking. In the UK, oncology treatment cannot be commenced without a biopsy-confirmed diagnosis. As such, those patients where a biopsy was the only surgical management were deemed to be managed conservatively. Inclusion criteria for the study comprised: confirmed histopathology diagnosis of GB, age 65 years or older, and surgical management, which was classified as either a burr hole biopsy or a craniotomy.
A retrospective review of all electronic patient notes was performed, and information collected included diagnosis, MGMT data, PS, oncological management, follow-up, and survival. No patients were treated without histological verification of GB, and all tissue was reviewed by a neurohistopathologist. There has not been a re-review of the tissue following the 2016 glioma classification. Routine MGMT analysis did not start in our unit until 2013 and therefore the data were missing for a number of patients. The type of surgery performed was determined from the operation notes and also by reviewing the postoperative imaging, where available. PS was measured prospectively according to ECOG/WHO PS classification[
Statistical methods
Initially, patient demographics were compared between the groups of surgical and oncological management using Fisher’s exact tests for nominal factors and Mann–Whitney U or Kruskal–Wallis tests for ordinal and continuous factors with two, or more than two categories, respectively. Patient survival from diagnosis was then assessed using Kaplan–Meier curves, with HRs produced using univariable Cox regression models. To test for a potential interaction between surgical and oncology management, a Cox regression model was produced with these two factors as covariates, as well as an interaction term. A multivariable Cox regression model was then produced, to identify factors that were independently associated with patient survival.
Analyses were also performed to identify factors associated with the change in PS between the pre- and post-operative periods. The change in status between these periods was divided into three categories: improved, no change, and worsened. A range of factors was then compared across these categories using Kendall’s tau or Kruskal–Wallis tests, for ordinal and nominal factors, respectively. A multivariable analysis was then performed using a binary logistic regression model, to identify independent predictors of an improved (vs. unchanged or worsened) PS postoperatively.
Analyses were then performed for the subgroup of patients for whom the MGMT status was recorded. Comparisons between those with methylated and unmethylated tumors were performed using Mann–Whitney U or Fisher’s exact tests, as applicable. Kaplan–Meier curves and univariable Cox regression analyses were performed separately for the subgroups that did and did not receive chemotherapy.
All analyses were performed using IBM SPSS 22 (IBM Corp. Armonk, NY), with p<0.05 deemed to be indicative of statistical significance throughout.
Ethics approval
This study was registered locally as an audit (CARMS reference number: 17875). Since it utilized pseudonymized data that were retrospectively extracted, formal ethics approval was not sought. This is in accordance with local research policies protocol and the Health Research Authority, UK.
RESULTS
Demographics
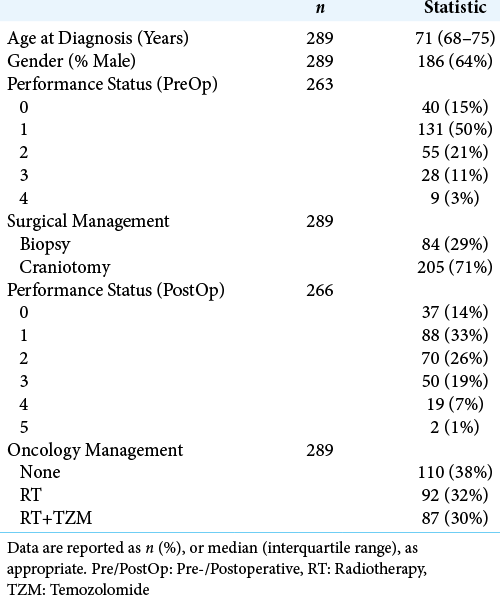
A total of n = 289 patients were identified, with a median age at diagnosis of GB of 71 years (interquartile range [IQR]: 68–75), and of whom the majority (64%) were male. Further patient demographics of the cohort are reported in
Surgical management
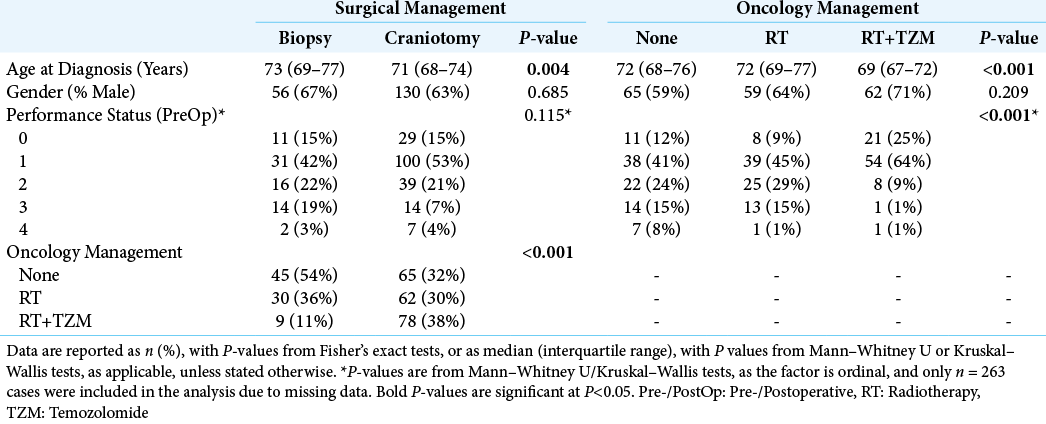
The majority of patients were managed with a craniotomy (71%, n = 205), with the remainder (29%, n = 84) having biopsies performed. In the craniotomy group, n = 165 patients had a postoperative scan from which the extent of resection (EOR) could be assessed; of these 70% (116/165) had >80% of the tumor volume resected. Surgical management was found to differ significantly by age at diagnosis, with patients undergoing craniotomies being younger than those treated with biopsies (median: 71 vs. 73 years, P = 0.004). No significant differences in gender distribution (P = 0.685) or preoperative PS (P = 0.115) were detected between the biopsy and craniotomy groups [
Oncology management
Oncology management was by RT alone in 32% (n = 92) and a combination of RT and TZM (RT+TZM) in 30% (n = 87), with the remainder receiving no oncological treatment (38%, n = 110). Patient age differed significantly between these groups (P < 0.001) [
Survival
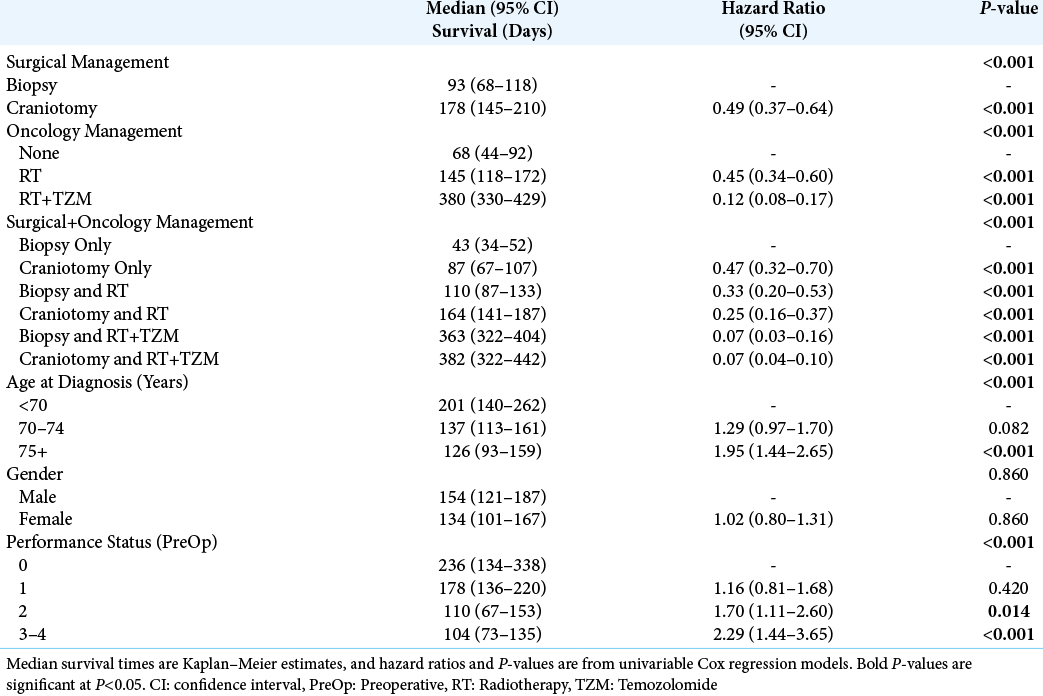
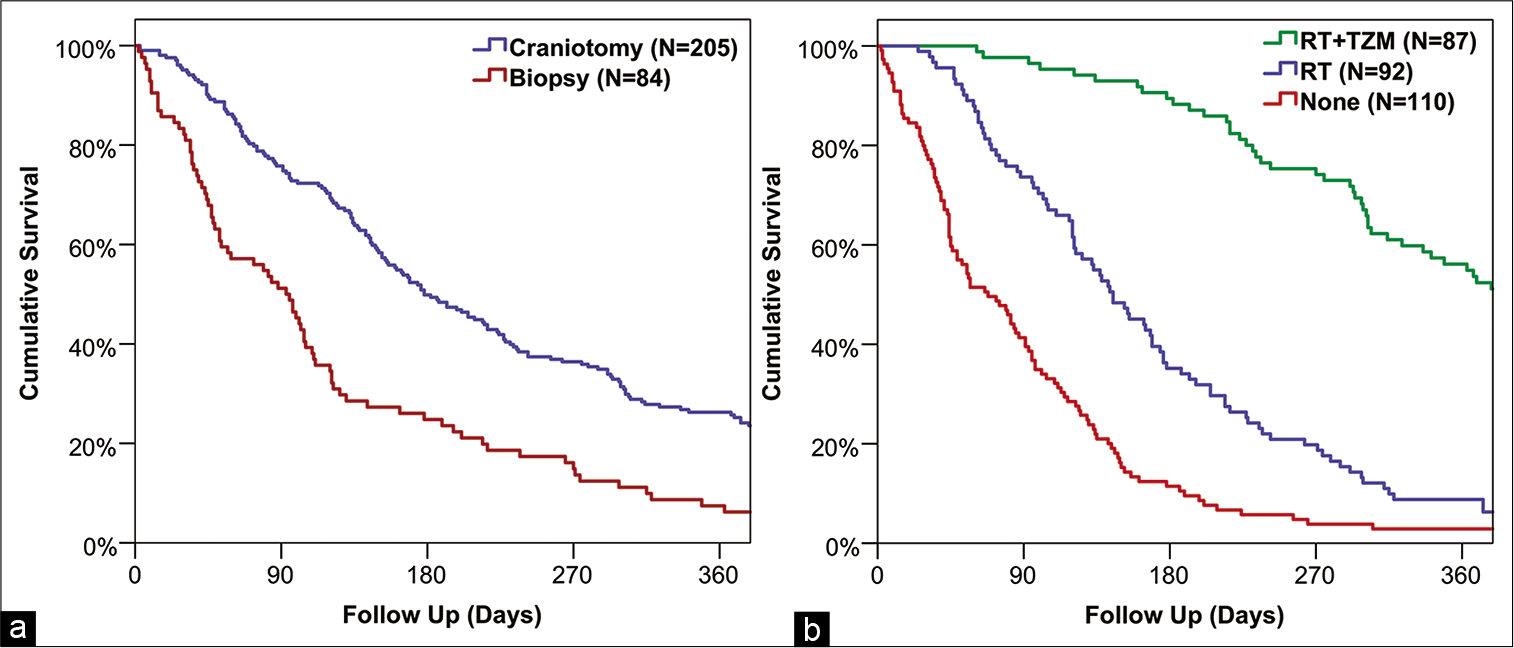
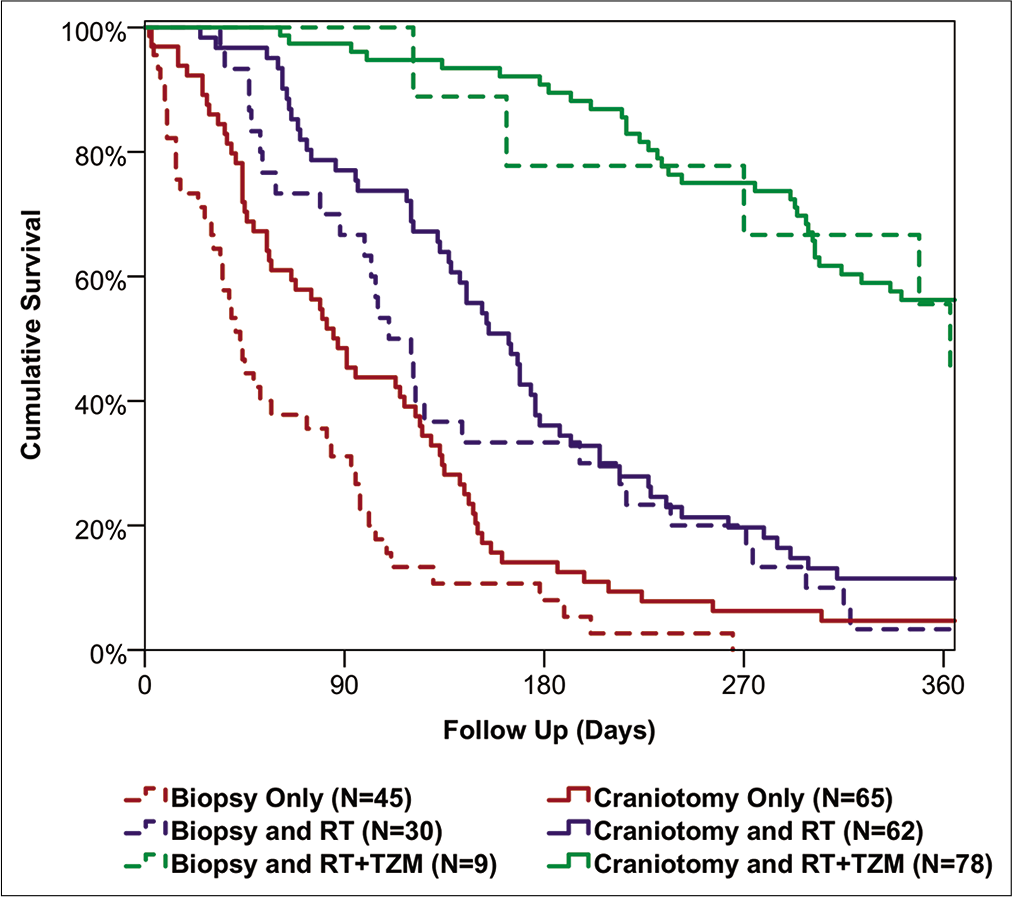
The median survival from diagnosis for the cohort as a whole was 148 days (95% CI: 128–168), and associations between factors and patient survival are reported in
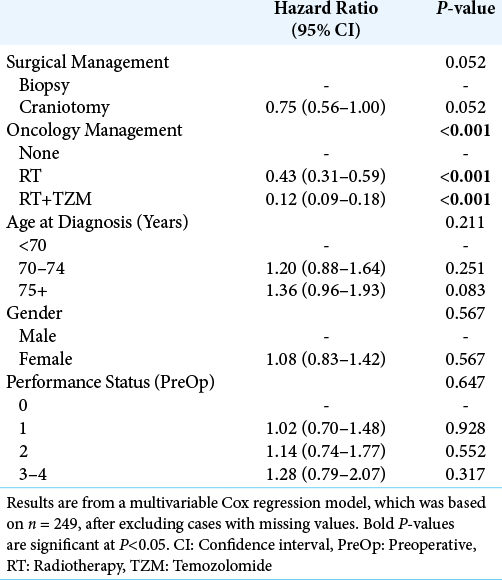
Of the other factors considered, survival was found to decline significantly with patient age at diagnosis (P < 0.001), from a median of 201 days in those aged <70 years to 126 days in those aged 75+ years. In addition, survival declined significantly with the PS score (P < 0.001), from a median of 236 days in those with a preoperative score of 0 to 104 days in those scoring 3–4. Since age had previously been found to be significantly associated with both surgical and oncology management, and PS score was associated with the latter, a multivariable analysis was then performed, to account for these potentially confounding factors [
PS
In the preoperative period, PS was recorded in n = 263 patients, of whom 15% (n = 40) had a PS of 0, with the most common status being 1 (50%, n = 131).
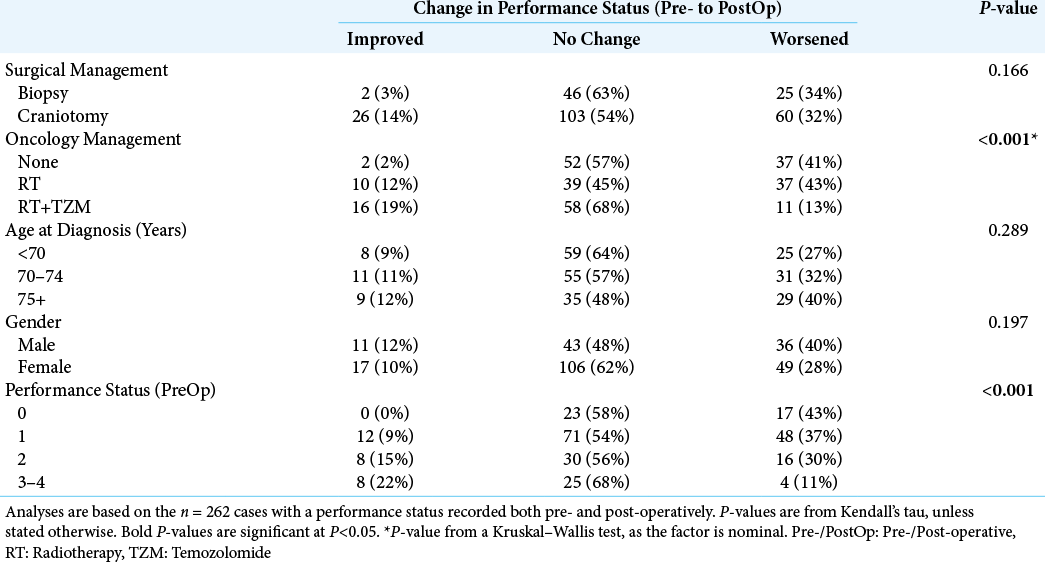
A total of n = 262 patients had a PS recorded both pre-and post-operatively. Of these, the PS was unchanged in the majority of patients (57%, n = 149), with improvements (i.e., reductions) in 11% (n = 28), and worsening (i.e., increases) in 32% (n = 85).
On univariable analysis [
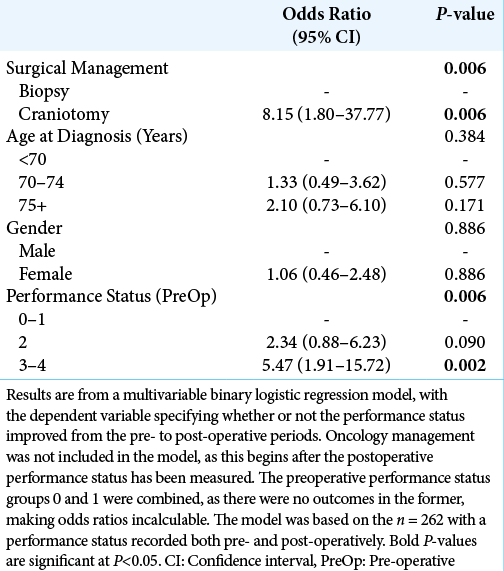
A multivariable analysis was then performed, to identify independent predictors of improvements in PS [
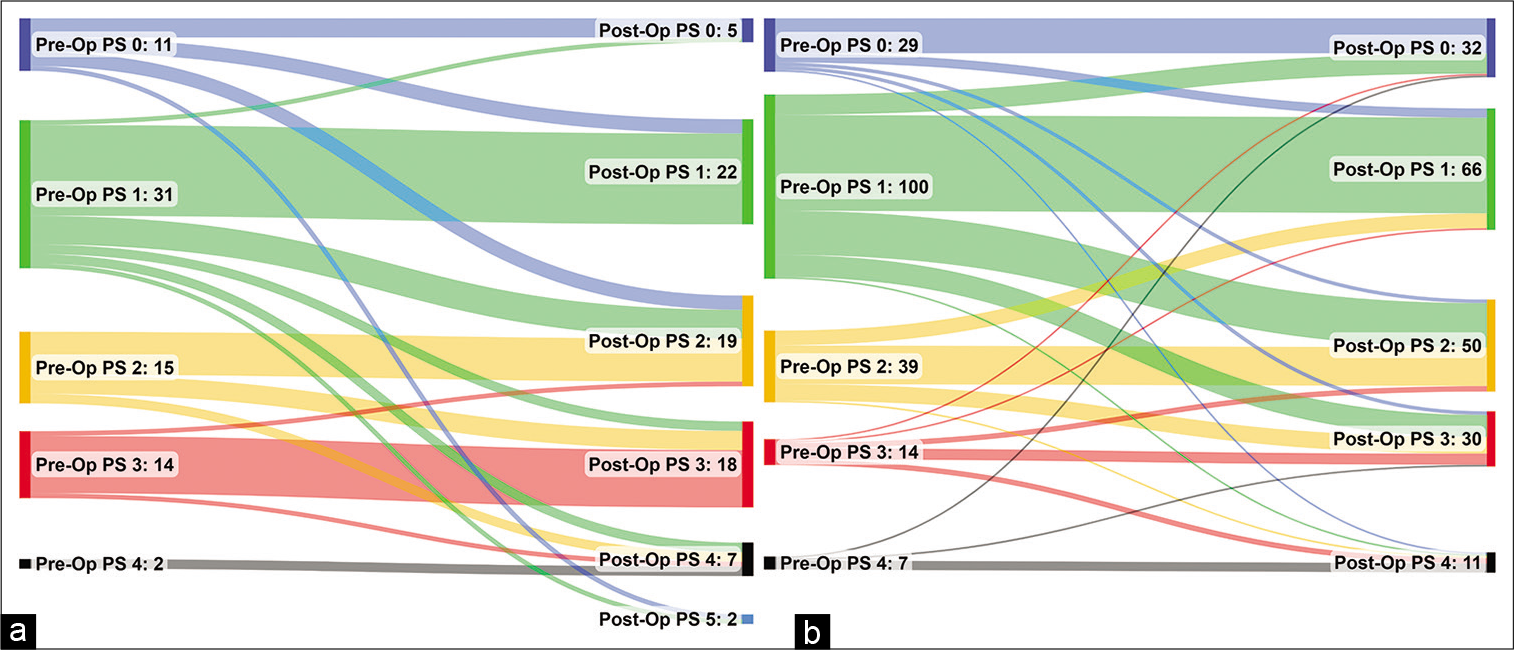
Figure 3:
Sankey diagram of the pre- to post-operative changes in performance status after (a) biopsy and (b) craniotomy. Patients with missing data for either the pre- or post-operative assessment are excluded, hence the plots are based on n = 73 for biopsy and n = 189 for craniotomy. Pre-/PostOp: Pre-/PostOperative, PS: performance status.
MGMT status
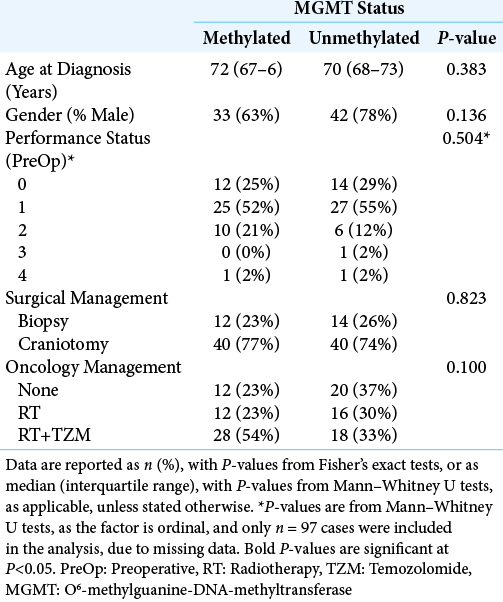
The MGMT status was only recorded in 106 patients (37%), of whom 52 (49%) had methylated tumors and 54 (51%) were unmethylated. Comparisons between these groups found no significant differences in demographic or treatment-related factors [
DISCUSSION
The current standard of care for GB, the Stupp protocol, includes maximal resection plus adjuvant TZM and RT, resulting in a mean survival of approximately 14 months.[
In our study, overall survival in elderly patients with GB was found to vary dramatically with the extent of treatment, from a median of 382 days in those receiving a craniotomy followed by combined RT and TZM, to just 43 days in those treated with biopsies and no further oncological management.
However, the major issue with comparing outcomes between treatments in a retrospective or non-randomized study design is the confounding effect of case selection. Patients treated with the most aggressive surgical and neuro-oncology management were found to be significantly younger than those receiving more conservative treatment; specifically, younger patients were more likely to be treated with craniotomy (vs. biopsy) and to receive combined TZM and RT. The previous studies have also highlighted that elderly populations are less likely to receive oncology treatment in general, which could be reflection of older patients being less amenable to aggressive interventions, due to lower physiological resilience and poorer preoperative PS.[
In an attempt to adjust for the confounding effects of age and PS, as well as to isolate the effects of surgical and oncology management, a multivariable analysis was performed. This found the approach to oncology management to be independently associated with overall survival, with combined RT and TZM leading to the best survival outcomes. After adjusting for effect of oncological management, the benefit of craniotomy (vs. biopsy) was found to be relatively small in comparison, and did not reach statistical significance in the final model. In addition, neither patient age nor preoperative PS was found to be significant independent predictors of overall survival in this model, implying that it is the largely the extent of oncological treatment, rather than surgical treatment, age or PS, that moderates survival for elderly patients with GB in our study.
While craniotomy was not found to be a significant independent predictor of overall survival, patients undergoing craniotomy were significantly more likely to see a postoperative improvement in PS, compared to those treated with biopsies. This was particularly pronounced in those with the poorest preoperative PS (i.e., scoring 3–4), where 33% of those receiving a craniotomy saw a postoperative improvement in PS, compared to 6% of those treated with biopsies. As such, while the direct benefit of craniotomy on patient survival was modest, the postcraniotomy improvement in PS may have allowed some patients who were previously unfit for aggressive oncological management to receive RT and TZM, indirectly improving their prognosis. The argument here would suggest that even patients with a poor PS should be considered for a craniotomy, provided the tumor is a suitable operative target. Other complementary treatments, such as corticosteroids, should also be considered and optimized, to give patients the best possible chance of tolerating aggressive surgical and oncological management.
The tradition of cytoreductive surgery is to some extent becoming less central to the management of GB with the identification of tumor biomarkers. MGMT methylation status is one of many such biomarkers, which is used to optimize treatment, with an aim to maximize patient survival. Unmethylated MGMT tumors are unlikely to respond to TZM, which was also observed in our study, with TZM offering no significant survival benefit in this subgroup of tumors. As such, there are clear benefits to maximizing the EOR (≥86%) in these unmethylated MGMT tumors.[
The “Glioma Cancer research in the 100,000 genome project” is a project that will be looking to find important genetic mutations that can help improve care and tailor treatment for patients diagnosed with GB. The project has started recruiting in the UK, and all age groups are eligible for enrollment. It will be interesting to see the results and what benefits it can bring to the elderly in particular with a GB diagnosis.[
To summarize, elderly patients will have a poorer PS compared to younger patients and, consequently, are less likely to receive aggressive interventions in the form of surgery, RT and chemotherapy.[
Limitations of this study
The primary limitation of the study was the potential for section bias and confounding, in light of the retrospective design. The surgical and oncological management approaches differed significantly by age and PS, which will have acted as confounding factors in the analysis. Multivariable analyses were performed, in an attempt to adjust for the effects of these factors, and isolate the independent effects of treatment. However, it is likely that some degree of residual confounding will be present, both due to confounders that were not considered and imperfect model fit, meaning that significant relationships can only be inferred to be indicative of associations with outcomes, rather than causal effects. In addition, the data were from a single center, and so may not be generalizable to other centers, particularly those with different patient demographics and treatment protocols.
CONCLUSION
Our retrospective data demonstrate that the elderly population with GB can derive significant benefit from aggressive oncology treatment with combined RT and TZM, regardless of surgical intervention. Whilst combining this with craniotomy resulted in the longest survival, the direct benefit over a simple biopsy was modest. However, craniotomy did lead to improved PS, which could subsequently increase the likelihood of a patient being deemed eligible for more aggressive oncological treatment. Therefore, every attempt should be made to offer these patients an optimal treatment, with all options taken into consideration. This degree of management requires close cooperation among neurosurgeons, anesthetists, neurooncologists, and neuropathologists to carefully select patients who are fit enough to benefit from this demanding treatment.
Declaration of patient consent
Patients’ consent not required as patients’ identities were not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. . Available from: https://www.ecog-acrin.org/resources/ecog-performance-status [Last accessed on 2022 May 08].
2. . Available from: https://www.genomicsengland.co.uk/research/glioma [Last accessed on 2022 May 08].
3. Babu R, Komisarow JM, Agarwal VJ, Rahimpour S, Iyer A, Britt D. Glioblastoma in the elderly: The effect of aggressive and modern therapies on survival. J Neurosurg. 2016. 124: 998-1007
4. Brodbelt A, Greenberg D, Winters T, Williams M, Vernon S, Collins VP. Glioblastoma in England: 2007-2011. Eur J Cancer. 2015. 51: 533-42
5. Brown TJ, Brennan MC, Li M, Church EW, Brandmeir NJ, Rakszawski KL. Association of the extent of resection with survival in glioblastoma: A systematic review and meta-analysis. JAMA Oncol. 2016. 2: 1460-9
6. Bruno F, Pellerino A, Palmiero R, Bertero L, Mantovani C, Garbossa D. Glioblastoma in the elderly: Review of molecular and therapeutic aspects. Biomedicines. 2022. 10: 644
7. Byun J, Kim YH, Nam SJ, Park JE, Cho YH, Kim HS. Comparison of survival between partial resection and biopsy for primary glioblastoma: A propensity score-matched study World Neurosurg. 2019. 121: e858-66
8. Cire B. World’s Older Population Grows Dramatically. Available from: https://www.nia.nih.gov/news/worlds-older-population-grows-dramatically [Last accessed on 2020 May 21].
9. D’Amico RS, Englander ZK, Canoll P, Bruce JN. Extent of resection in glioma-a review of the cutting edge. World Neurosurg. 2017. 103: 538-49
10. Dolecek TA, Propp JM, Stroup NE, Kruchko C. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2005-2009. Neuro Oncol. 2012. 14: v1-49
11. Hanna C, Lawrie TA, Rogozinska E, Kernohan A, Jefferies S, Bulbeck H. Treatment of newly diagnosed glioblastoma in the elderly: A network meta-analysis. Cochrane Database Syst Rev. 2020. 3: CD013261
12. Heiland D, Haaker G, Watzlawik R, Delev D, Masalha W, Franco P. One decade of glioblastoma multiforme surgery in 342 elderly patients: What have we learned?. J Neurooncol. 2018. 140: 385-91
13. Keles GE, Anderson B, Berger MS. The effect of extent of resection on time to tumor progression and survival in patients with glioblastoma multiforme of the cerebral hemisphere. Surg Neurol. 1999. 52: 371-9
14. Kreth FW, Thon N, Simon M, Westphal M, Schackert G, Nikkhah G. Gross total but not incomplete resection of glioblastoma prolongs survival in the era of radiochemotherapy. Ann Oncol. 2013. 24: 3117-23
15. Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, DeMonte F. A multivariate analysis of 416 patients with glioblastoma multiforme: Prognosis, extent of resection, and survival. J Neurosurg. 2001. 95: 190-8
16. Lapierre N, Weller M, Stupp R, Perry JR, Brandes AA, Wick W. Optimal management of elderly patients with glioblastoma. Cancer Treat Rev. 2013. 39: 350-7
17. Laws ER, Parney IF, Huang W, Anderson F, Morris AM, Asher A. Survival following surgery and prognostic factors for recently diagnosed malignant glioma: Data from the Glioma outcomes project. J Neurosurg. 2003. 99: 467-73
18. Malmstrom A, Gronberg BH, Marosi C, Stupp R, Frappaz D, Schultz H. Temozolamide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: The Nordic randomised, phase 3 trial. Lancet Oncol. 2012. 13: 916-26
19. Marko NF, Weil RJ, Schroeder JL, Lang FF, Suki D, Sawaya RE. Extent of resection of glioblastoma revisited: personalized survival modeling facilitates more accurate survival prediction and supports a maximum-safe-resection approach to surgery. J Clin Oncol. 2014. 32: 774-82
20. McGirt MJ, Chaichana KL, Gathinji M, Attenello FJ, Than K, Olivi A. Independent association of extent of resection with survival in patients with malignant brain astrocytoma. J Neurosurg. 2009. 110: 156-62
21. Morgan E, Norman A, Laing K, Seal MD. Treatment and outcomes for glioblastoma in elderly compared with non-elderly patients: A population-based study. Curr Oncol. 2017. 24: e92-8
22. Oken M, Creech R, Tormey D, Horton J, Davis TE, McFadden ET. Toxicity and response criteria of the Eastern cooperative oncology group. Am J Clin Oncol. 1982. 5: 649-55
23. Perry JR, Laperriere N, O’Callaghan CJ, Brandes AA, Menten J, Phillips C. Short-course radiation plus temozolomide in elderly patients with glioblastoma. N Engl J Med. 2017. 376: 1027-37
24. Pessina F, Navarria P, Cozzi L, Rudà R, Nibali MC, Simonelli M. Is surgical resection useful in elderly newly diagnosed glioblastoma patients? Outcome evaluation and prognostic factors assessment. Acta Neurochirurg. 2018. 160: 1779-87
25. Roa W, Brasher P, Bauman G, Anthes M, Bruera E, Chan A. Abbreviated course of radiation therapy in older patients with glioblastoma mulitforme: A prospective randomized clinical trial. J Clin Oncol. 2004. 22: 1583-8
26. Roth P, Gramatzki D, Weller M. Management of elderly patients with glioblastoma. Curr Neurol Neurosci Rep. 2017. 17: 35
27. Sales A, Bette S, Barz M, Huber T, Wiestler B, Ryang YM. Role of postoperative tumor volume in patients with MGMTunmethylated glioblastoma. J Neurooncol. 2019. 142: 529-36
28. Sanai N, Berger MS. Glioma extent of resection and its impact on patient outcome. Neurosurgery. 2008. 62: 753-66
29. Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg. 2011. 115: 3-8
30. Scott JG, Suh JH, Elson P, Barnett GH, Vogelbaum MA, Peereboom DM. Aggressive treatment is appropriate for glioblastoma multiforme patients 70 years old or older: A retrospective review of 206 cases. Neuro Oncol. 2011. 13: 428-36
31. Sharma M, Bellamkonda S, Mohapatra S, Meola A, Jia X, Mohammadi A. Correlation between the residual tumor volume, extent of tumor resection, and O6-methylguanine DNA methyltransferase status in patients with glioblastoma. World Neurosurg. 2018. 116: e147-61
32. Smrdel U, Vidmar MS, Smedel A. Glioblastoma in patients over 70 years of age. Radiol Oncol. 2018. 52: 167-72
33. Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncol. 2006. 7: 392-401
34. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005. 352: 987-96
35. Youssef M, Ludmir E, Mandel J, Patel AJ, Jalali A, Treiber J. Treatment strategies for glioblastoma in older patients: Age is just a number. J Neurooncol. 2019. 145: 357-64