- Department of Neurosurgery, School of Medicine, Loma Linda University, Loma Linda, California, United States.
- Department of Radiology, Loma Linda University Medical Center, Loma Linda, California, United States.
- Department of Neurosurgery, Loma Linda University School of Medicine, Loma Linda, California, United States.
Correspondence Address:
Miguel Angel Lopez-Gonzalez, Department of Neurosurgery, Loma Linda University School of Medicine, Loma Linda, California, United States.
DOI:10.25259/SNI_542_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Emmanuel Omosor1, Elena Milosavljevic2, Edward Lawson1, Miguel Angel Lopez-Gonzalez3. Isolated cervical Cutibacterium acnes osteomyelitis in a patient with no primary source of infection – A case report and review of the literature. 06-Oct-2023;14:358
How to cite this URL: Emmanuel Omosor1, Elena Milosavljevic2, Edward Lawson1, Miguel Angel Lopez-Gonzalez3. Isolated cervical Cutibacterium acnes osteomyelitis in a patient with no primary source of infection – A case report and review of the literature. 06-Oct-2023;14:358. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=12578
Abstract
Background: Cervical vertebral osteomyelitis (CVO) is a rare pathology that leads to progressive osseous degradation and eventual loss of bone putting the patient at risk of devastating neurological injury in the event of bony collapse or instability. Cutibacterium acnes formerly called Propionibacterium acnes is rare, but within the last two decades has been an increasingly reported cause of osteomyelitis. The majority of C. acnes vertebral osteomyelitis cases have been reported in patients with a history of prior invasive procedures where direct contamination at the time of procedure was suspected as the underlying etiology.
Case Description: We report a unique case of an otherwise healthy 39-year-old male with no prior history of invasive procedures who presented with CVO secondary to C. acnes. He underwent surgical debridement and fusion in conjunction with antibiotic treatment. The patient recovered well and a 2-year follow-up with serial imaging showed no evidence of disease recurrence.
Conclusion: C. acnes is an under-recognized and under-reported etiology of spine infections. Clinicians should be aware of the pathological potential and atypical presentation of C. acnes vertebral osteomyelitis.
Keywords: Cervical vertebral osteomyelitis, Cutibacterium acnes, Spine surgery
INTRODUCTION
Cervical vertebral osteomyelitis (CVO) is a rare pathology that leads to progressive osseous degradation and eventual loss of the infected neck bone.[
CVO has a reported incidence of about 3–11% of all osteomyelitis cases involving the spine.[
Cutibacterium acnes formerly called Propionibacterium acnes is a nonspore-forming anaerobic Gram-positive bacillus that is also found in skin microflora[
In this article, we report a unique case of an otherwise healthy 39-year-old male with no prior history of invasive procedures who presented with CVO secondary to C. acnes.
CASE PRESENTATION
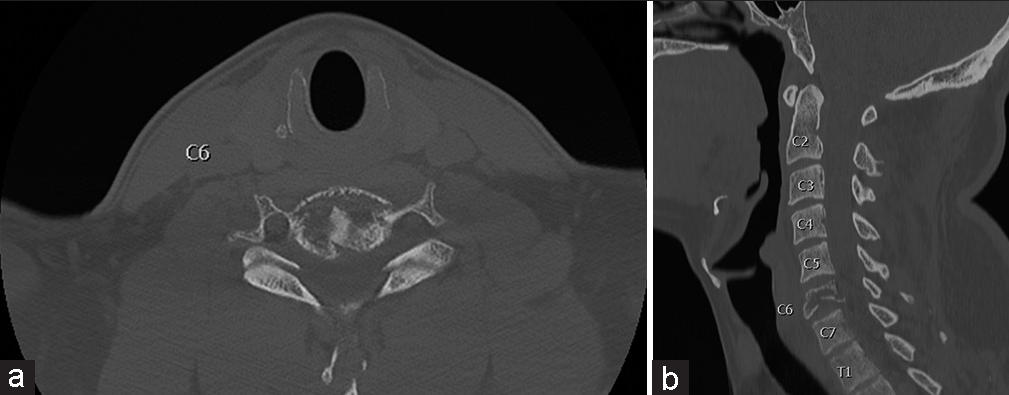
A 39-year-old male with no previous history of invasive procedures or primary C. acnes infection presented to the emergency department 1 week after the insidious onset of severe posterior neck and back pain, muscle spasms, and bilateral hand numbness. The patient was afebrile but initial labs were remarkable for mildly elevated leukocytes (12.26 × 109/L) and significantly elevated C-reactive protein (CRP) (7.8 mg/L). The patient was started on cefazolin after cultures were drawn. A computed tomography (CT) scan showed comminuted compression deformity of the C6 vertebral body with 50–55% height loss and retropulsion of bony fragments into the spinal canal resulting in moderate thecal sac narrowing. There was no underlying lytic lesion to suggest a neoplastic process and no gross paraspinal soft-tissue abnormality was noted [
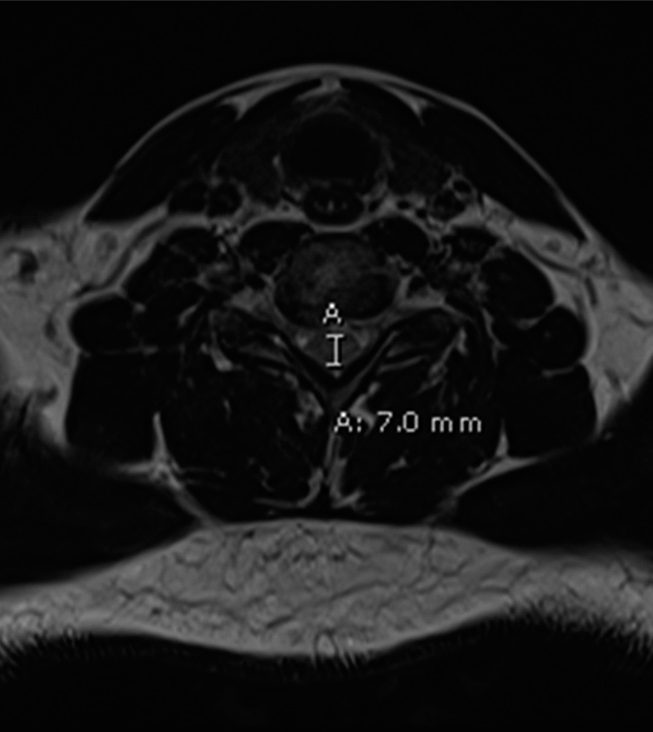
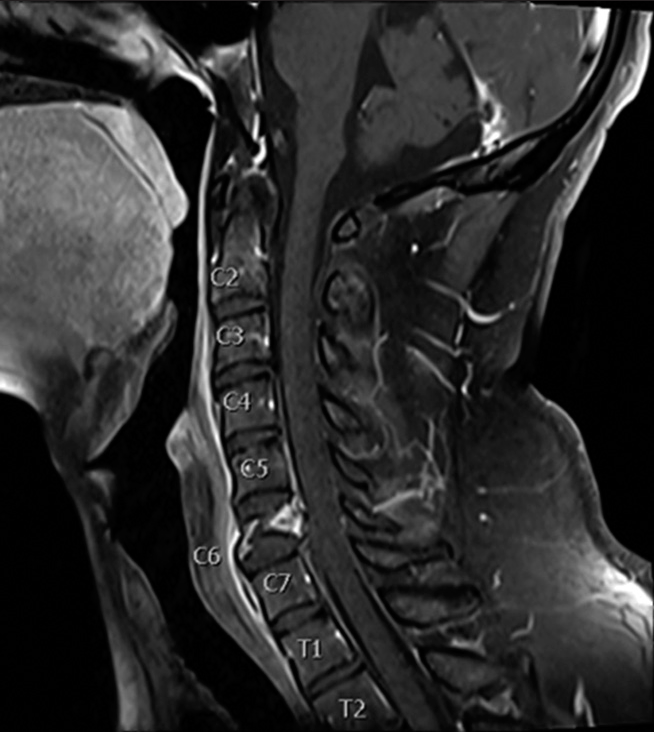
On magnetic resonance imaging (MRI), there was a redemonstration of a comminuted C6 compression fracture with retropulsion of bone fragments into the spinal canal resulting in a thecal sac anterior-posterior width of 7 mm with patency of the bilateral neural foramina [
The patient underwent a C6 corpectomy followed by C5-C7 anterior cervical fixation. The patient tolerated the procedure well and the post-operative physical exam was at baseline. Intraoperative cultures and polymerase chain reaction confirmed the diagnosis of C. acnes osteomyelitis. The patient was discharged with a 14-day course of Cefalexin.
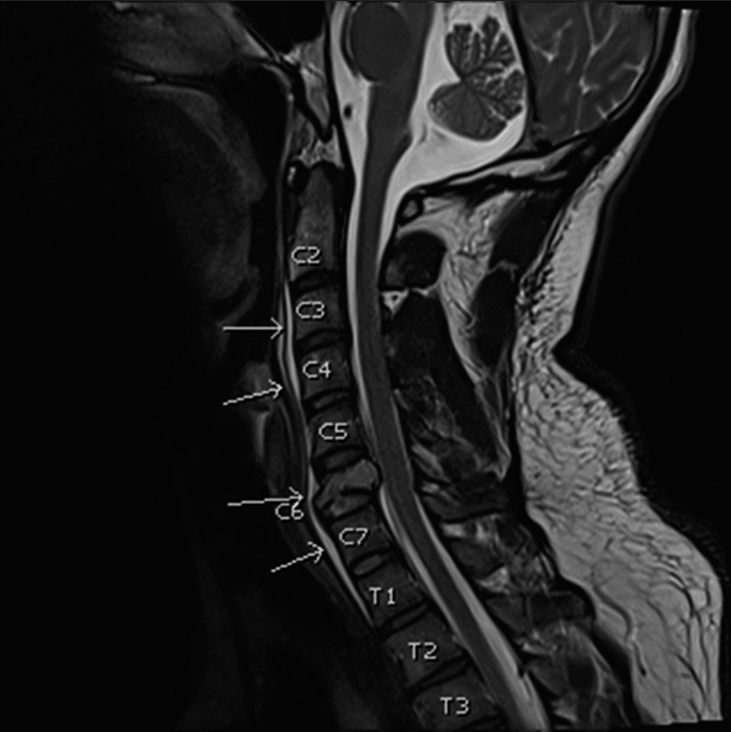
The patient was subjected to a comprehensive monitoring period spanning 2 years, during which both CT and MRI examinations consistently revealed the absence of any recurring infection with stable postsurgical changes related to C6 corpectomy and C5-C7 anterior fixation. These findings serve as evidence of successful antibiotic treatment and eradication of the infectious microbe. In addition, the patient has fully recovered their physical strength and sensation in all limbs, and any previously experienced pain has completely resolved.
DISCUSSION
While C. acnes vertebral osteomyelitis appears to occur almost exclusively in patients who have undergone prior invasive procedures,[
There are several unique attributes of C. acnes vertebral osteomyelitis as compared to other pyogenic causes of vertebral osteomyelitis. The first is that while most patients diagnosed with C. acnes vertebral osteomyelitis initially present with the nonspecific complaint of back pain, these symptoms are rarely accompanied by fever, elevated CRP, and ESR, and almost always have a history of a previous spine procedure.[
There is no current standardized, evidence-based guideline for the management of C. acnes CVO.[
Passerini et al. conducted a study on nonhardware-associated vertebral osteomyelitis, examining its characteristics and outcomes.[
CONCLUSION
The laboratory and radiographic findings of C. acnes nonimplant-associated vertebral osteomyelitis are not well-characterized. However, what is known is that they often present with atypical findings. Due to the slow-growing nature of these bacteria in anaerobic culture, C. acnes is likely an under-recognized and under-reported etiology of spine infections. Clinicians should be aware of the pathological potential and atypical presentation of C. acnes vertebral osteomyelitis.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The author(s) confirms that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Acosta FL, Chin CT, Quiñones-Hinojosa A, Ames CP, Weinstein PR, Chou D. Diagnosis and management of adult pyogenic osteomyelitis of the cervical spine. Neurosurg Focus. 2004. 17: E2
2. Barnes B, Alexander JT, Branch CL. Cervical osteomyelitis: A brief review. Neurosurg Focus. 2004. 17: E11
3. Carragee EJ. Pyogenic vertebral osteomyelitis. J Bone Joint Surg Am. 1997. 79: 874-80
4. Hahn BS, Kim KH, Kuh SU, Park JY, Chin DK, Kim KS. Surgical treatment in patients with cervical osteomyelitis: Single institute’s experiences. Korean J Spine. 2014. 11: 162-8
5. Jha Y, Chaudhary K. Diagnosis and treatment modalities for osteomyelitis. Cureus. 2022. 14: e30713
6. Kavanagh N, Ryan EJ, Widaa A, Sexton G, Fennell J, O’Rourke S. Staphylococcal osteomyelitis: Disease progression, treatment challenges, and future directions. Clin Microbiol Rev. 2018. 31: e00084-17
7. Khalil JG, Gandhi SD, Park DK, Fischgrund JS. Cutibacterium acnes in spine pathology: Pathophysiology, diagnosis, and management. J Am Acad Orthop Surg. 2019. 27: e633-40
8. Kowalski TJ, Berbari EF, Huddleston PM, Steckelberg JM, Osmon DR. Propionibacterium acnes vertebral osteomyelitis: Seek and ye shall find?. Clin Orthop Relat Res. 2007. 461: 25-30
9. Lutz MF, Berthelot P, Fresard A, Cazorla C, Carricajo A, Vautrin AC. Arthroplastic and osteosynthetic infections due to Propionibacterium acnes: A retrospective study of 52 cases, 1995-2002. Eur J Clin Microbiol Infect Dis. 2005. 24: 739-44
10. Noble RC, Overman SB. Propionibacterium acnes osteomyelitis: Case report and review of the literature. J Clin Microbiol. 1987. 25: 251-4
11. Otto M. Staphylococcus colonization of the skin and antimicrobial peptides. Expert Rev Dermatol. 2010. 5: 183-95
12. Passerini M, Maamari J, Geno Tai DB, Patel R, Tande AJ, Temesgen Z. Cutibacterium acnes in spine tissue: Characteristics and outcomes of non-hardware-associated vertebral osteomyelitis. J Bone Jt Infect. 2023. 8: 143-9
13. Schimmer RC, Jeanneret C, Nunley PD, Jeanneret B. Osteomyelitis of the cervical spine: A potentially dramatic disease. J Spinal Disord Tech. 2002. 15: 110
14. Serushan M, Spencer DL, Yeh WL, Kaminski M, Skosey JL. Osteomyelitis of cervical spine from Propionibacterium acnes. Arthritis Rheum. 1982. 25: 346-8
15. Toyoshima H, Tanaka K, Tanigawa M, Masuda N, Ishiguro C, Tanaka H. Vertebral osteomyelitis caused by the novel pathogen Cutibacterium modestum: A case report. BMC Infect Dis. 2022. 22: 305
16. Tsai CE, Lee FT, Chang MC, Yu WK, Wang ST, Liu CL. Primary cervical osteomyelitis. J Chin Med Assoc. 2013. 76: 640-7
17. Uçkay I, Dinh A, Vauthey L, Asseray N, Passuti N, Rottman M. Spondylodiscitis due to Propionibacterium acnes: Report of twenty-nine cases and a review of the literature. Clin Microbiol Infect. 2010. 16: 353-8
18. Walter G, Kemmerer M, Kappler C, Hoffmann R. Treatment algorithms for chronic osteomyelitis. Dtsch Arztebl Int. 2012. 109: 257-64