- Department of Neurosurgery, University of Oklahoma, Oklahoma City, United States.
- Department of Neurosurgery, Oklahoma University Health Science Center, Oklahoma City, United States.
Correspondence Address:
Benjamin Joseph Lee, Department of Neurosurgery, University of Oklahoma, Oklahoma, United States.
DOI:10.25259/SNI_685_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Benjamin Joseph Lee1, Lance Villeneuve2, Michael Martin2. Laminectomy as treatment for abrupt neurological decline in acrodysostosis: A case report. 21-Oct-2022;13:476
How to cite this URL: Benjamin Joseph Lee1, Lance Villeneuve2, Michael Martin2. Laminectomy as treatment for abrupt neurological decline in acrodysostosis: A case report. 21-Oct-2022;13:476. Available from: https://surgicalneurologyint.com/surgicalint-articles/11950/
Abstract
Background: Acrodysostosis (ACRO) is a rare disorder of peripheral bone development which can be either sporadic or inherited with mutations in the PRKAR1A or PDE4D genes. The resulting phenotypical characteristics are variable and overlap with other dysostosis conditions, making diagnosis difficult without genotyping. Vertebral malformations have been reported with ACRO resulting in slowly progressive spinal cord compression leading to radiculopathy or myelopathy.
Case Description: A 19-year-old female diagnosed with ACRO presented with progressively worsening lower extremity paraparesis, sensory loss, and urinary retention; she was wheelchair-bound. A magnetic resonance imaging showed cord signal change at the T2/T3 levels with accompanying diffuse cord edema between T6-T8. Six months following a T2/T3 and T6/T7 laminectomy, the patient’s symptoms improved, but she still required a wheelchair.
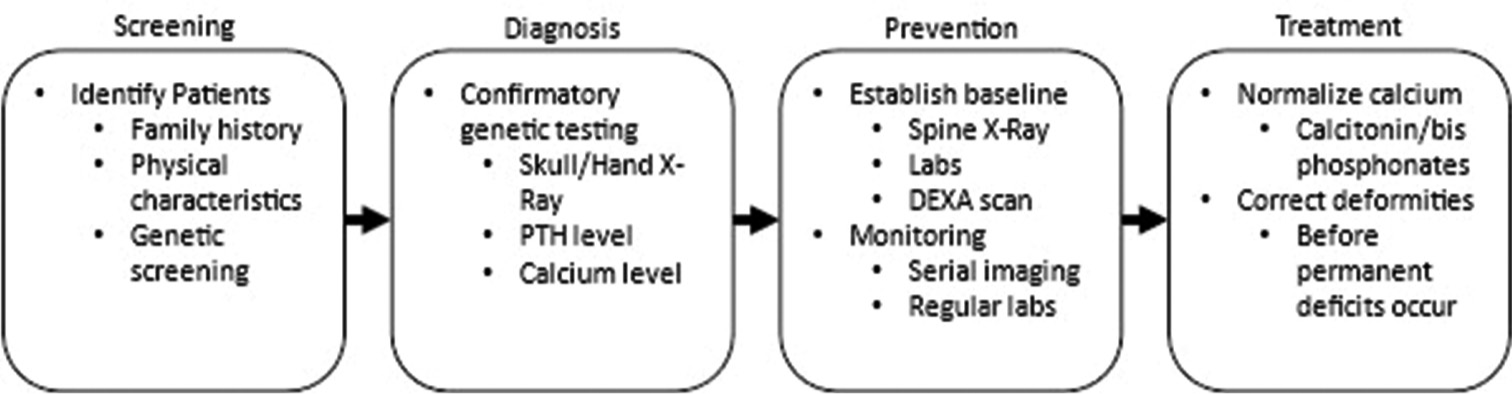
Conclusion: Patients with ACRO should be regularly monitored for cord compression to allow for early surgical decompression to prevent long-term, devasting neurological compromise.
Keywords: Acrodysostosis, Laminectomy, Neurosurgery
INTRODUCTION
Acrodysostosis (ACRO) is a disorder in peripheral bone development initially described in 1968 by Maroteaux.[
CASE DESCRIPTION
A 19-year-old female with a history of ACRO presented with a 3-month complaint of progressive paraparesis leading to paraplegia, a loss of pinprick sensation at the T4 level, and urinary retention. Magnetic resonance imaging showed central canal stenosis that was more prominent on the left, cord signal change at the T2/T3 level [
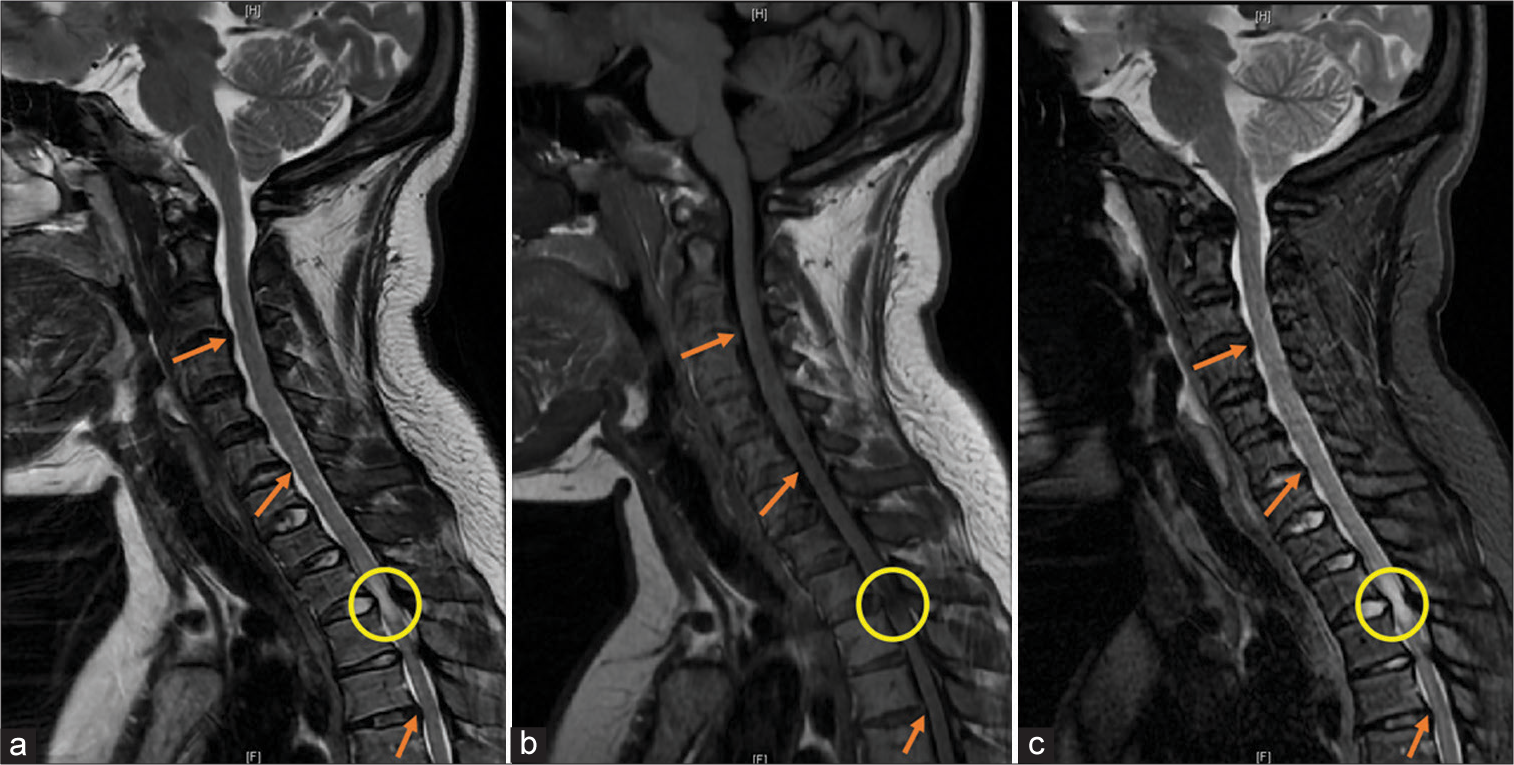
Figure 1:
(a) Sagittal T2-weighted magnetic resonance imaging (MRI) of the cervical spine showing a hyperintensity and buckling of ligamentum flavum (circle) at T2-T3 and canal stenosis (arrows) at multiple levels. (b) Sagittal T1-weighted MRI showing hypointensity and buckling of the ligamentum flavum (circle) at T2-T3 and canal stenosis (arrows) at multiple levels. (c) Sagittal short T1-weighted inversion recovery (STIR) MRI showing hyperintensity and buckling of the ligamentum flavum (circle) at T2-T3 consistent with cord edema and canal stenosis (arrow) at multiple levels.
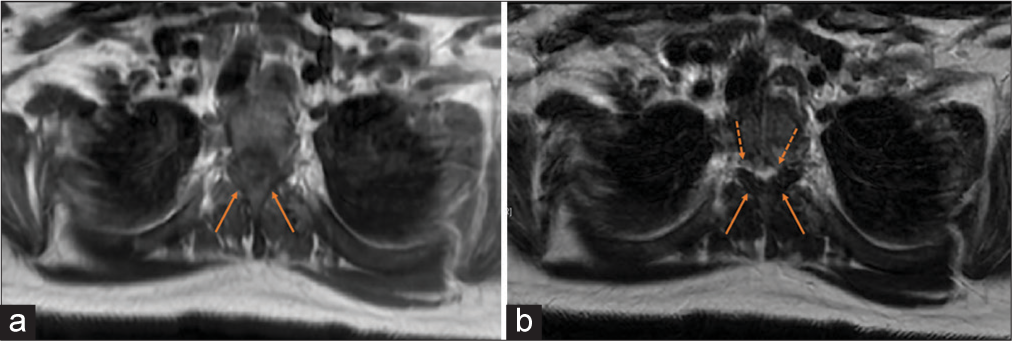
Figure 2:
(a) Axial T1-weighted FLAIR MRI at T2/T3 level showing thickening/hypertrophy of ligamentum flavum (solid arrows) and decreased spinal canal diameter. (b) Axial T2-weighted MRI at the T2/T3 level showing thickening/hypertrophy of ligamentum flavum (solid arrows) and cord signal change (dashed arrows) moreprominent on the left than the right.
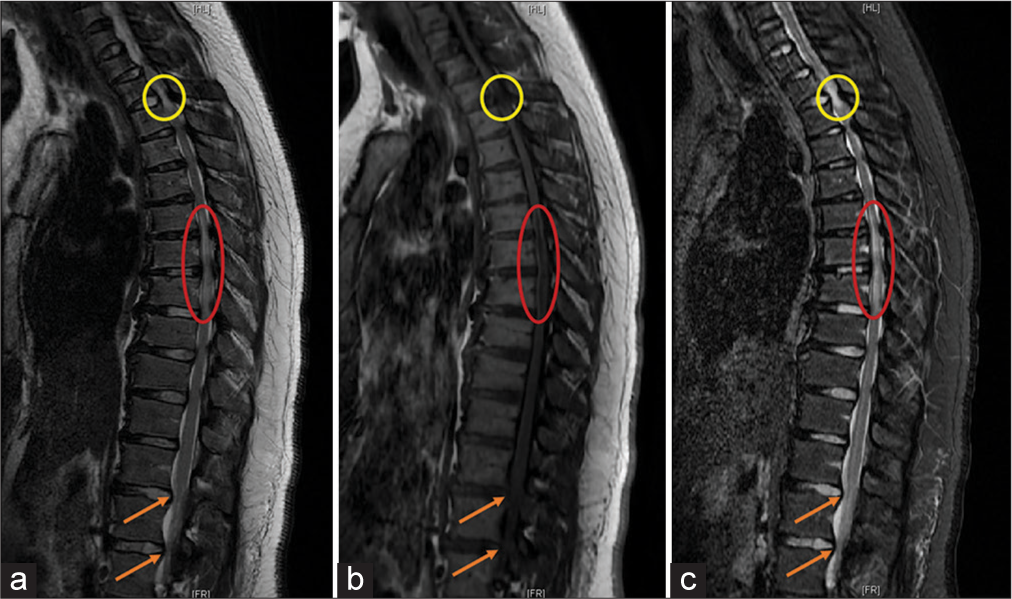
Figure 3:
(a) Sagittal T2-weighted MRI showing a hyperintensity of the cord and buckling of ligamentum flavum (circle) at T2-T3, diffuse hyperintensity (oval) from T6-T8, and lumbar stenosis (arrows). (b) Sagittal T1-weighted MRI showing hypointensity of the cord and buckling of the ligamentum flavum (circle) at T2-T3, diffuse hypointensity (oval) from T6-T8, and lumbar stenosis (arrows). (c) Sagittal short T1-weighted inversion recovery (STIR) MRI showing hyperintensity and buckling of the ligamentum flavum (circle) at T2-T3 consistent with cord edema, diffuse hyperintensity (oval) from T6-T8, and lumbar stenosis (arrow).
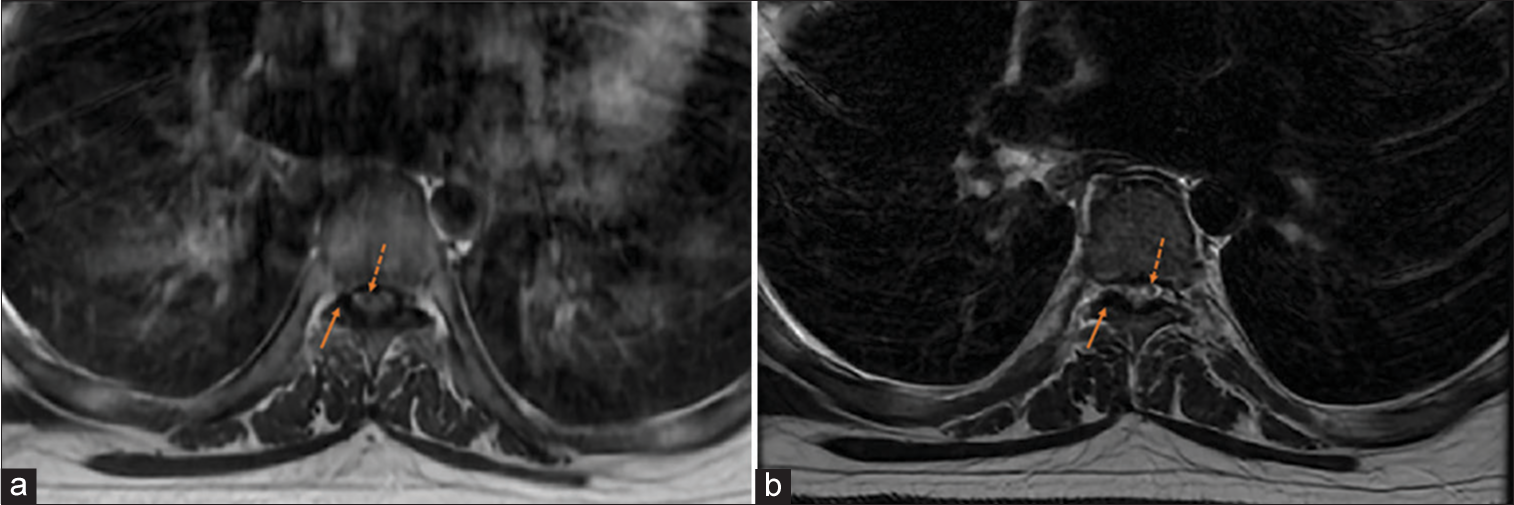
Figure 4:
(a) Axial T1-weighted FLAIR MRI at T6/T7 level showing thickening/hypertrophy of ligamentum flavum (solid arrows) and slight cord change (dashed arrows). (b) Axial T2-weighted MRI at T6/T7 level showing thickening/hypertrophy of ligamentum flavum (solid arrows) and slight cord change (dashed arrows).
DISCUSSION
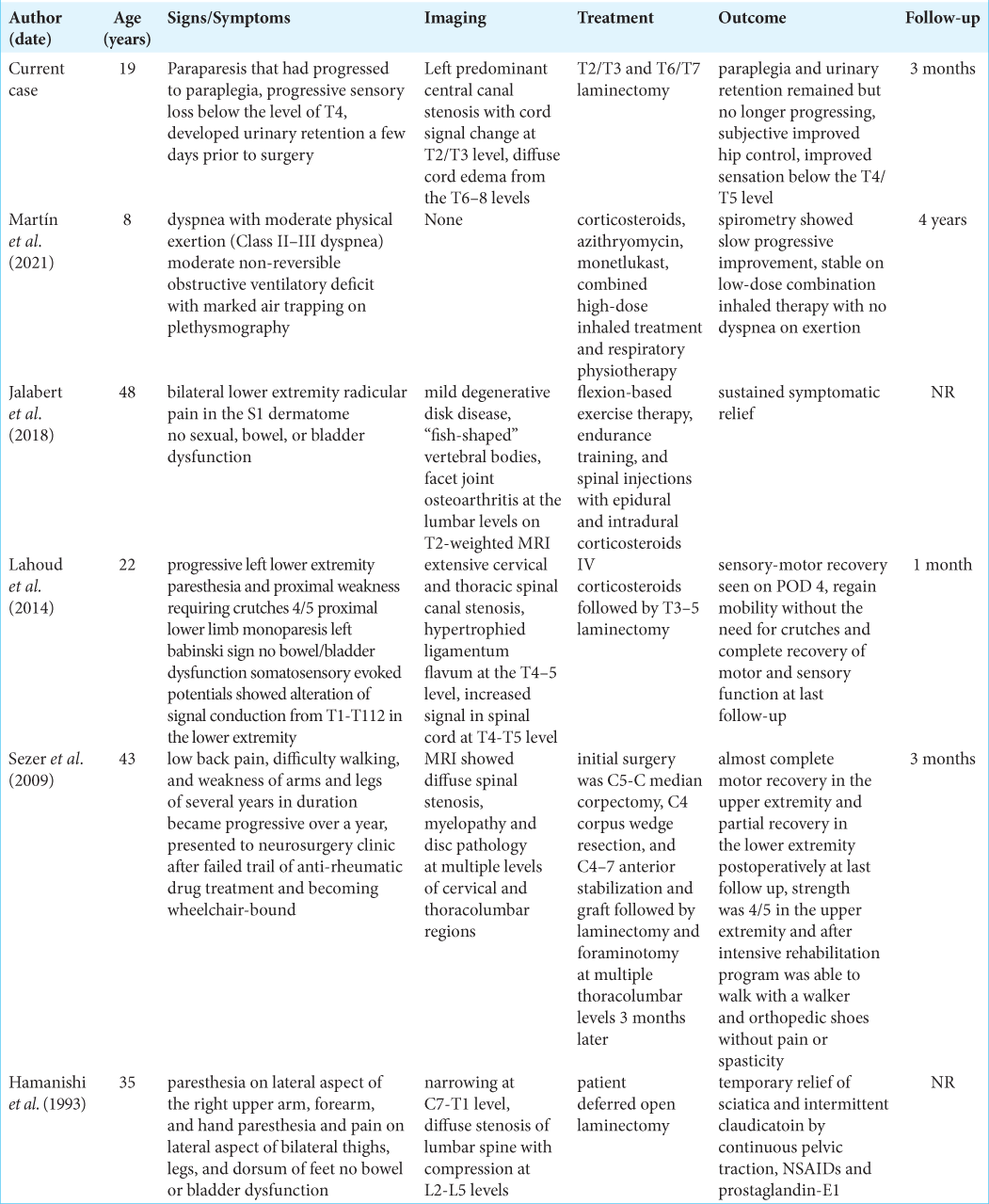
We found only five instances of ACRO that were myelopathic and attributable to MR-documented stenosis. [
Acrodysotosis: Differential diagnostic considerations
The differential diagnostic considerations for ACRO include idiopathic hypercalcemia, pseudohypoparathyroidism, and ankylosing spondylitis (AS).[
CONCLUSION
Patients with ACRO, either sporadic or genetic in origin, should be routinely monitored for the onset of radiculopathy and/or myelopathy reflecting progressive cord compromise due to stenosis, which may or may not warrant surgical spinal decompression.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
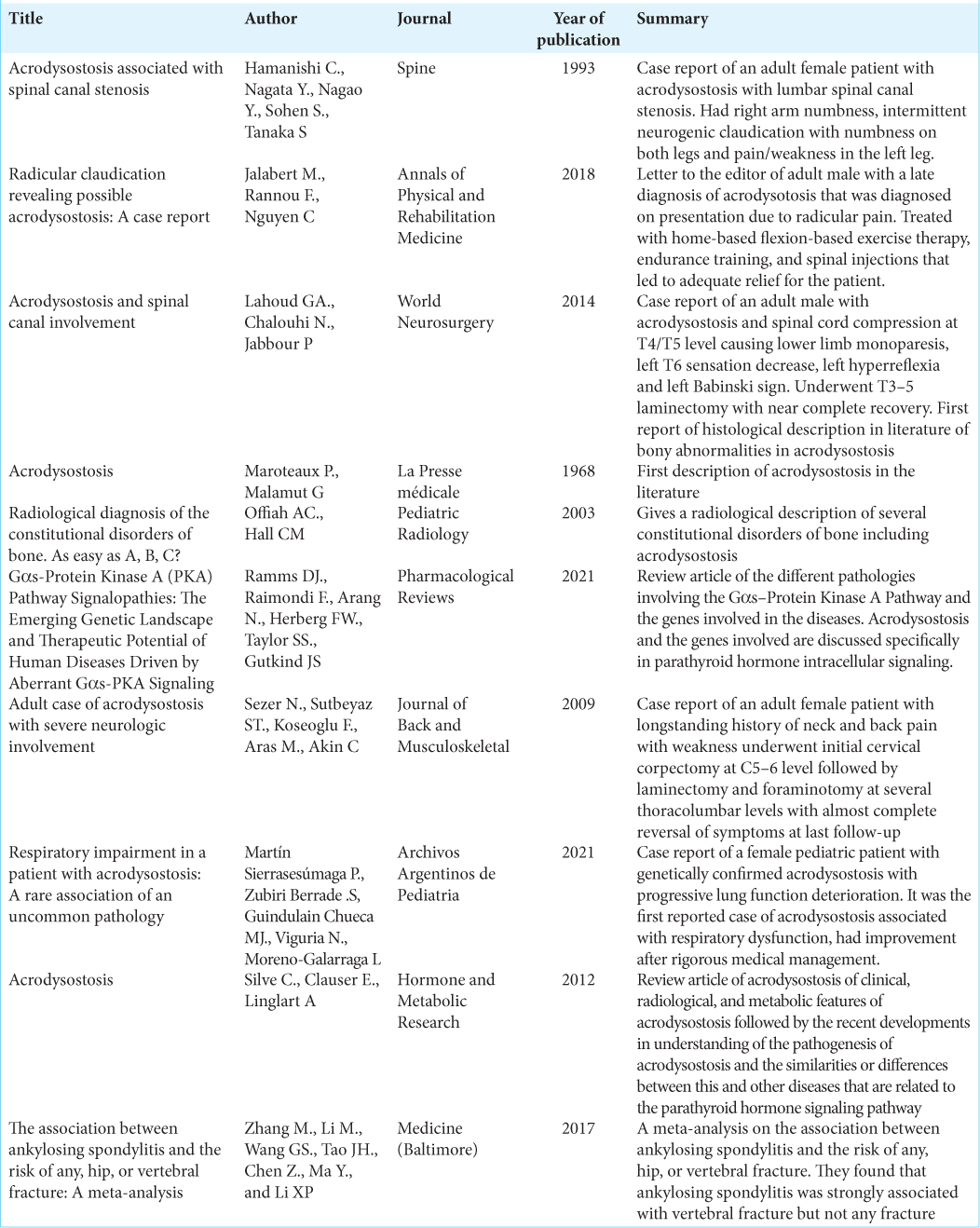
1. Hamanishi C, Nagata Y, Nagao Y, Sohen S, Tanaka S. Acrodysostosis associated with spinal canal stenosis. Spine (Phila Pa 1976). 1993. 18: 1922-5
2. Jalabert M, Rannou F, Nguyen C. Radicular claudication revealing possible acrodysostosis: A case report. Ann Phys Rehabil Med. 2018. 61: 60-1
3. Lahoud GA, Chalouhi N, Jabbour P. Acrodysostosis and spinal canal involvement. World Neurosurg. 2014. 82: 537.e539-11
4. Maroteaux P, Malamut G. Acrodysostosis. Presse Med. 1968. 76: 2189-92
5. Martín PS, Zubiri SB, Guindulain MJ, Viguria N, MorenoGalarraga L. Respiratory impairment in a patient with acrodysostosis: A rare association of an uncommon pathology. Arch Argent Pediatr. 2021. 119: e340-4
6. Offiah AC, Hall CM. Radiological diagnosis of the constitutional disorders of bone, As easy as A, B, C?. Pediatr Radiol. 2003. 33: 153-61
7. Ramms DJ, Raimondi F, Arang N, Herberg FW, Taylor SS, Gutkind JS. Gαs-protein kinase A (PKA) pathway signalopathies: The emerging genetic landscape and therapeutic potential of human diseases driven by aberrant Gαs-PKA signaling. Pharmacol Rev. 2021. 73: 155-97
8. Sezer N, Sutbeyaz ST, Koseoglu F, Aras M, Akin C. Adult case of acrodysostosis with severe neurologic involvement. J Back Musculoskelet Rehabil. 2009. 22: 125-9
9. Silve C, Clauser E, Linglart A. Acrodysostosis. Horm Metab Res. 2012. 44: 749-58
10. Zhang M, Li XM, Wang GS, Tao JH, Chen Z, Ma Y. The association between ankylosing spondylitis and the risk of any, hip, or vertebral fracture: A meta-analysis. Medicine (Baltimore). 2017. 96: e8458