- Department of Neurosurgery, Yamagata City Hospital Saiseikan, Japan
- Department of Emergency Medicine, Yamagata City Hospital Saiseikan, Japan
- Department of Neurosurgery, Yamagata University, Yamagata, Japan.
Correspondence Address:
Atsushi Kuge, Department of Emergency Medicine and Neurosurgery, Yamagata City Hospital, Saiseikan, Yamagata, Japan.
DOI:10.25259/SNI_37_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Kazuki Nakamura1, Atsushi Kuge1,2, Tetsu Yamaki1, Yu Shimokawa1, Masahiro Tanaka1, Shinjiro Saito1, Rei Kondo1, Yukihiko Sonoda3. Late-onset aqueductal membranous occlusion treated neuroendoscopic procedure and consideration of its pathological findings: A case report. 24-Mar-2023;14:98
How to cite this URL: Kazuki Nakamura1, Atsushi Kuge1,2, Tetsu Yamaki1, Yu Shimokawa1, Masahiro Tanaka1, Shinjiro Saito1, Rei Kondo1, Yukihiko Sonoda3. Late-onset aqueductal membranous occlusion treated neuroendoscopic procedure and consideration of its pathological findings: A case report. 24-Mar-2023;14:98. Available from: https://surgicalneurologyint.com/surgicalint-articles/12215/
Abstract
Background: Aqueduct of Sylvius stenosis/obstruction interferes with cerebrospinal fluid (CSF) flow and leads to the non-communicating hydrocephalus. Acquired non-neoplastic causes of aqueduct of Sylvius stenosis/ obstruction include simple stenosis, gliosis, slit-like stenosis, and septal formation, but the detailed mechanisms are not clear. In the present study, we experienced a case of late-onset aqueductal membranous occlusion (LAMO) successfully treated by neuroendoscopic procedure, which allowed us to examine the pathology of the membranous structures of the aqueduct of Sylvius occlusion.
Case Description: A 66-year-old woman presented with gradually progressive gait disturbance, cognitive dysfunction, and urinary incontinenc. Brain magnetic resonance imaging (MRI) showed enlargement of the bilateral lateral ventricles and the third ventricle without dilatation of fourth ventricle, and heavily T2-weighted images showed an enlarged aqueduct of Sylvius and a membranous structure at its caudal end. Gadolinium contrast-enhanced T1-weighted images showed no neoplastic lesions. We diagnosed this case that the hydrocephalus due to late-onset idiopathic aqueductal stenosis or LAMO and the patient underwent endoscopic third ventriculostomy and endoscopic aqueduct oplasty. Membranous tissue specimens were obtained from the occluded aqueduct of Sylvius at the time of treatment. Histopathological examination revealed gliosis, and inside the gliosis, there were cell clusters that appeared to be ependymal cells and were corpora amylacea. We confirmed CSF flow at the site of obstruction of the aqueduct of Sylvius and the stoma of the third ventricle floor by MRI images. Her symptoms were improved immediately.
Conclusion: We experienced a case of LAMO successfully treated by neuroendoscopic procedure, which allowed us to examine the pathology of the membranous structure of the aqueduct of Sylvius. The pathological study of LAMO is rare, and we report it, including a review of the literature.
Keywords: Hydrocephalus, Late-onset aqueductal membranous occlusion (LAMO), Neuroendoscopic surgery, Pathology
INTRODUCTION
The aqueduct of Sylvius is one of the narrowest pathways of cerebrospinal fluid (CSF), and its obstruction can be a cause of hydrocephalus. Late-onset aqueductal membranous occlusion (LAMO) is known as a non-neoplastic and acquired disorder of the aqueduct of Sylvius.[
CASE REPORT
A 66-year-old woman presented with progressively worsening gait disturbance and urinary incontinence over a year. She was diagnosed with hydrocephalus and was admitted to our department for further examinations and treatment.
She had a history of hypertension, hyperlipidemia, and cholecystitis, and no history of head trauma, encephalitis, or meningitis and other hemorrhagic events that would cause hydrocephalus.
Neurological findings revealed no consciousness disturbance, but slow overall movement, and a wide-base open-leg gait. Timed up and go test was 18.9 s. The preoperative mini-mental state examination (MMSE) score was 20/30 and the frontal assessment battery (FAB) score was 10/18. There was no motosensory disturbance, and rigidity, tremor, or head enlargement.
CSF study meant color: Watery clear, initial pressure: 15 cm/H2O, cell count: <1/uL, PH: 7.4, protein:30 (10–40) mg/dL, sugar:73 (50–75) md/dL, and chloride:113 (110–125) mEq/L. Blood analysis showed no inflammatory findings.
Brain magnetic resonance imaging (MRI) showed enlarged lateral and third ventricles without the dilatation of fourth ventricle. Narrowing of the subarachnoid space in the parietal region (high cranial area and longitudinal fissure) and heterogeneous enlargement of the basilar and Sylvian fissures indicated idiopathic normal pressure hydrocephalus was not observed [
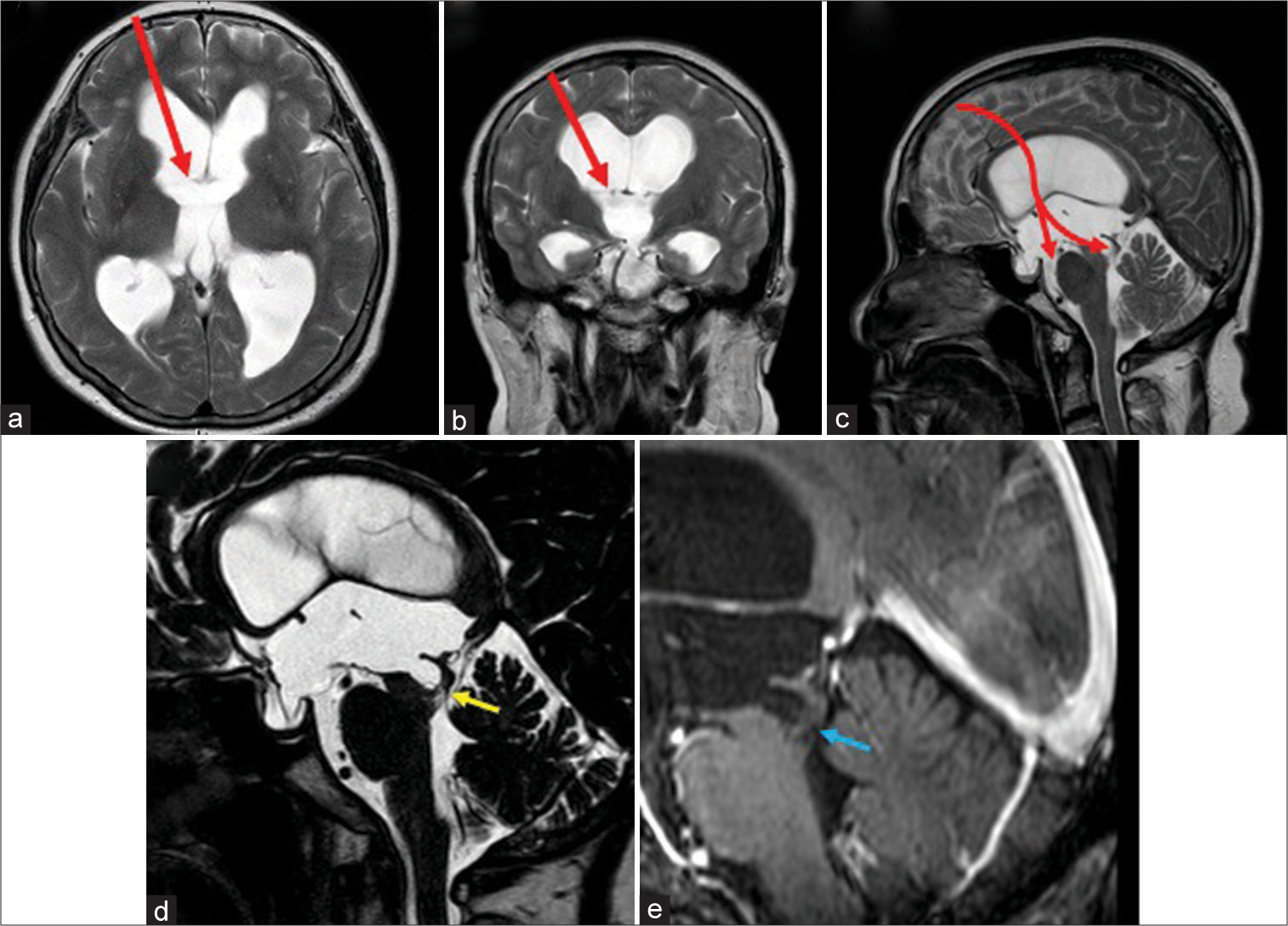
Figure 1:
Magnetic resonance imaging pretreatment findings: (a-c) T2WI showed ventricular dilatation of the lateral and the third ventricles but not of the fourth ventricle. Preoperative planning of the endoscopic corridor was shown as red arrow. (d) Heavily T2WI showed an enlarged aqueduct of Sylvius and a membranous structure at its caudal end (yellow arrow). (e) T1WI with gadolinium did not demonstrate any enhanced lesion (blue arrow).
We diagnosed with hydrocephalus caused stenosis or obstruction of aqueduct of Sylvius.
Preoperatively, endoscopic third ventriculostomy (ETV) alone was considered, but MRI showed a funnel-shaped enlargement of the entrance to the aqueduct of Sylvius with an obstructive mechanism at its base, and intraoperatively, it was recognized that the membrane structure in the obstructed area was very thin, so we decided to perform aqueduct plasty as well. When performing the procedure, we took great care to avoid mesencephalic injury using small forceps. Our endoscopic surgical corridor planned preoperatively from the anterior horn of the right lateral ventricle to the third ventricle floor and the aqueduct of Sylvius was presented [
Hence, we performed ETV, and endoscopic aqueduct plasty (EAP) as below. Postoperative MRI showed a reduction in ventricular size [
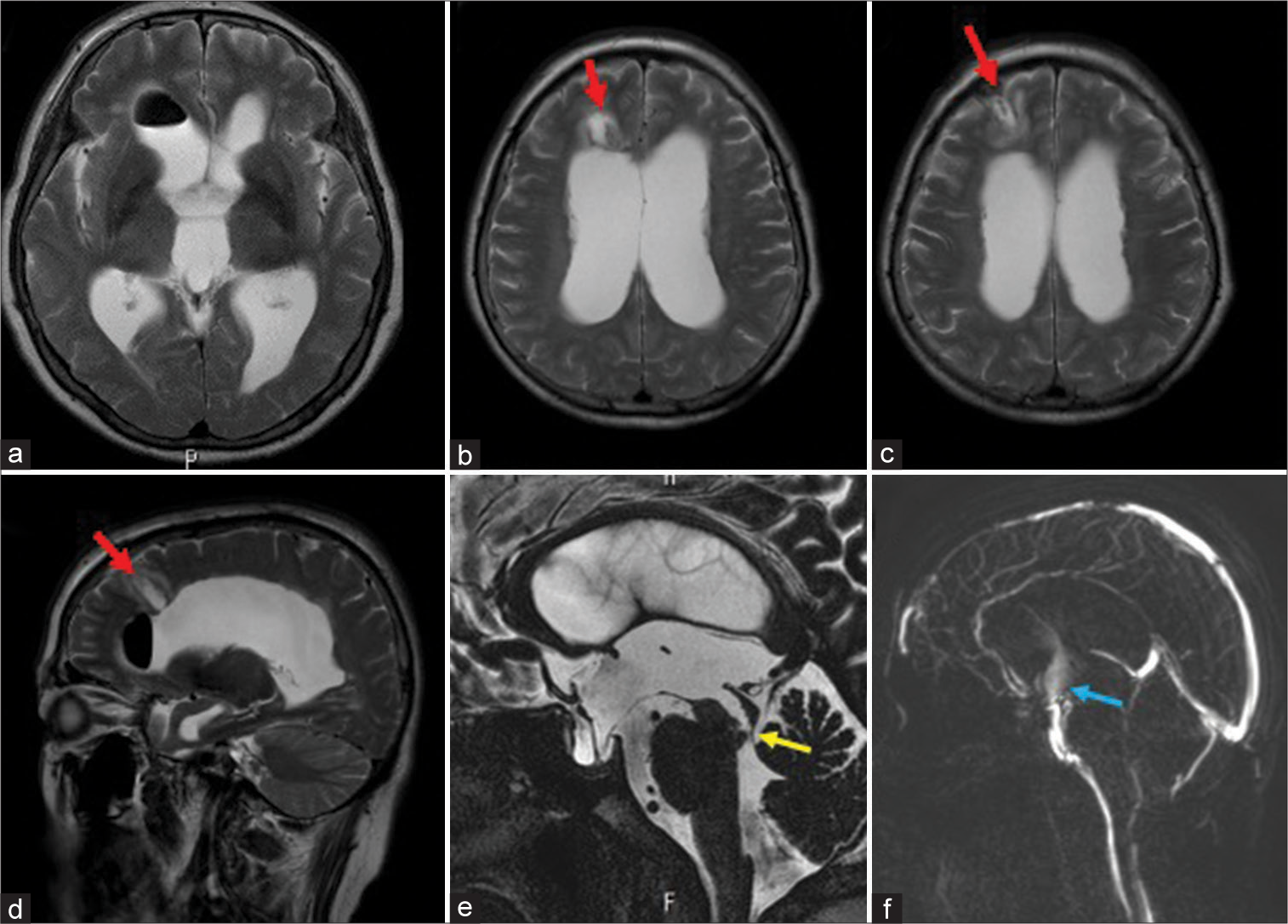
Figure 2:
Magnetic resonance imaging (MRI) posttreatment findings: (a-d) T2WI axial and saggitalimages showed mild ventricular shrinkage and was seen surgical collider at the right frontal lobe (red arrow). (e) Heavily T2WI showed the opening of the aqueduct of Sylvius (yellow arrow). (f) Phase contrast cine MRI showed cerebrospinal fluid flow at the stoma of the third ventricle floor (blue arrow).
Intraoperative findings
The flexible endoscope (Olympus VEF type V, Olympus, Tokyo, Japan) was inserted through anterior horn of the right lateral ventricle through a transparent sheath (Neuroport mini, Hakko, Tokyo, Japan). An enlarged lateral ventricle, foramen of Monro, third ventricle, and stretched interthalamic adhesion were observed, and the septum pellucidum was partially perforated [
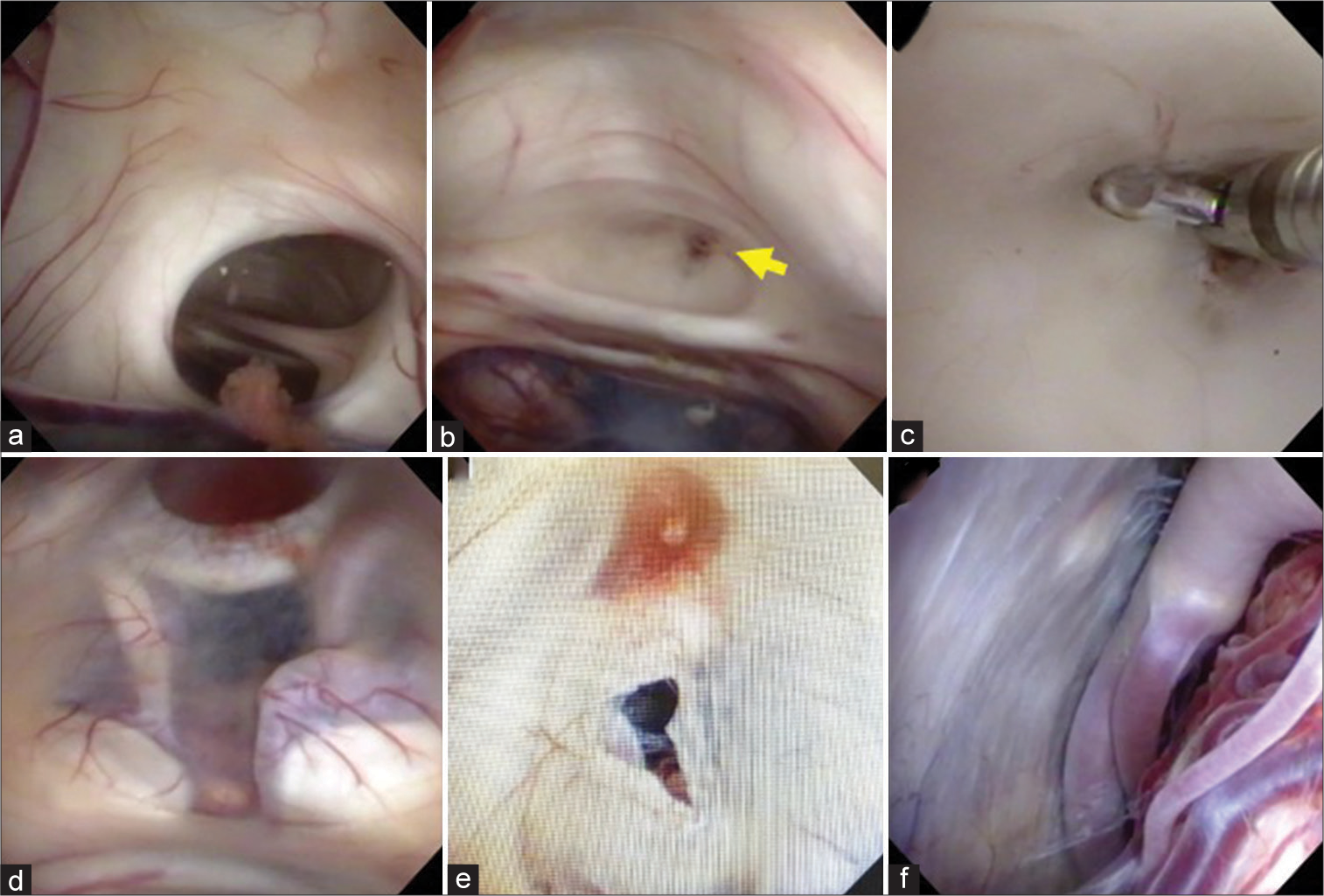
Figure 3:
Intraoperative findings (a) foramen of Monro was dilated and observed stretched interthalamic adhesion. (b) Membranous structure (yellow arrow) occluded the aqueduct of Sylvius. (c) Membranous structure was removed with forceps. (d) The floor of the third ventricle was thinning (e) A stoma was created at the floor of the third ventricle. (f) The Lilliquist’s membrane was thinning and defects in prepontine cistern.
Histopathological findings
Histopathologically, we examined the thin membranous structure that occluded the aqueduct of Sylvius. Hematoxylin Eosin: Staining revealed a mild increase of glia cells in the neuropil, reflecting gliosis. Within the cellular matrix, cell aggregates with an island-like distribution and loss of continuity which were observed [
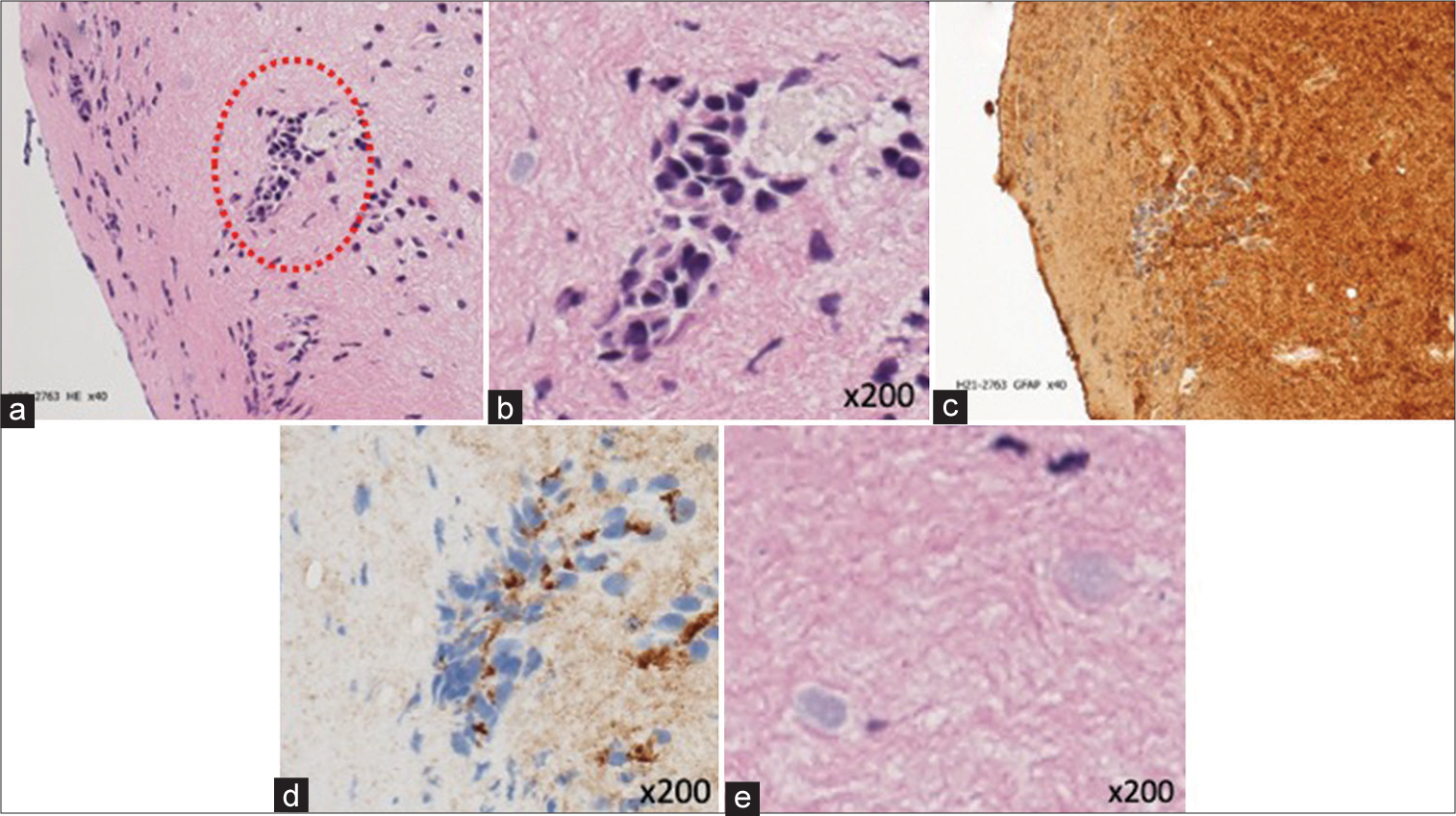
Figure 4:
Pathological findings (a and b) island-like cell aggregates with loss of continuity were seen in the neuropil on staining (red dotted circle). (c and d) Cells forming island-like aggregates were glial fibrillary acidic protein (d) positive and epithelial membrane antigen (d) positive. (e) Corpora amylacea were scattered in neuropil on staining.
DISCUSSION
LAMO is known as a disease that causes hydrocephalus due to non-neoplastic aqueduct of Sylvius occlusion. The disease concept was first proposed by Matsuda et al. in 2011,[
The common clinical presentation of these disease groups is often headache in young-onset cases and symptoms similar to the clinical presentation of normal pressure hydrocephalus in the elderly. The previous reports have reported the usefulness of sagittal and coronal sections of heavily T2-weighted images for imaging evaluation of membranous structures.[
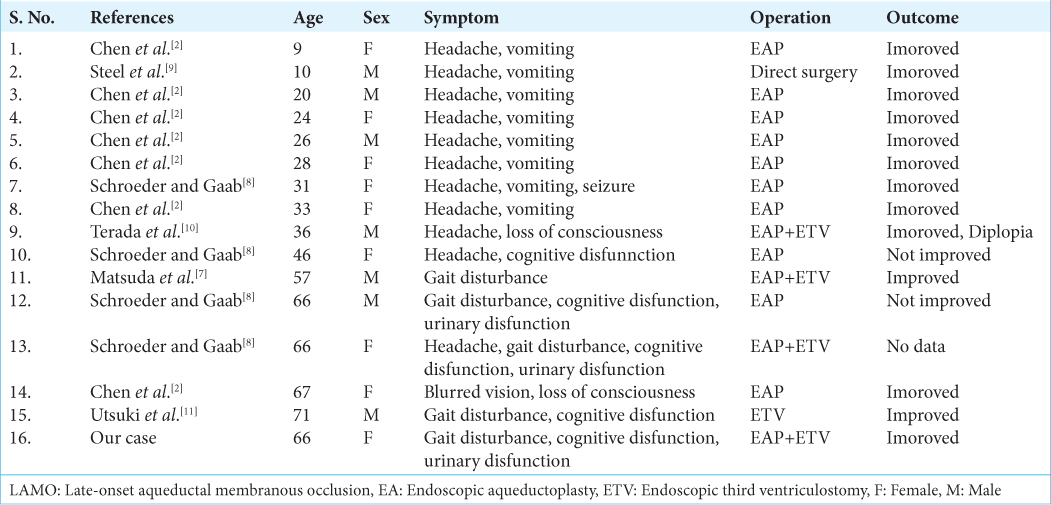
Fifteen cases of LAMO have been reported[
As far as we could find, there were no reports of detailed pathological examination about the membranous structures. The exact mechanism of formation of the septa that occlude the aqueduct of Sylvius is unknown. In this case, we were able to histopathologically examine the membranous structure that formed in the aqueduct of Sylvius.
Histopathologically, membranous structure in the aqueduct of Sylvius showed increased glia cells, reflecting gliosis, and cell aggregates of ependymal cells. In addition, Corpora amylacea, which are clumps of polyglucosamine that accumulate at the tips of astrocyte projections, were found in the neuropil. In the previous reports, Del Biggio[
CONCLUSION
We experienced a case of LAMO successfully treated by neuroendoscopic procedure, which allowed us to examine the pathology of the membranous structure of the aqueduct of Sylvius. The histopathological findings suggested that the result of ependymal repair failure may have contributed to the obstruction of the aqueduct of Sylvius.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Cavanagh JB. Corpora-amylacea and the family of polyglucosan diseases. Brain Res Rev. 1999. 29: 265-5
2. Chen G, Zheng J, Xiao Q, Liu Y. Application of phase-contrast cine magnetic resonance imaging in endoscopic aqueductoplasty. Exp Ther Med. 2013. 5: 1643-8
3. Del Biggio MR. Ependymal cells: Biology and pathology. Acta Neuropathol. 2010. 119: 55-73
4. Flora N, Kulaselaren N, Mudali SK, Swaminathan T. Compensated aqueduct of sylvius obstruction by web-a case report. Indian J Radiol Imaginng. 2005. 15: 19-20
5. Fukuhara T, Luciano MG. Clinical features of late-onset idiopathic aqueductal stenosis. Surg Neurol. 2001. 55: 132-7
6. Marta R, Jaume DV, Elisabet A, Jordi V, Carme P. From corpora amylacea to wasteosomes: History and perspectives. Ageing Res Rev. 2021. 72: 101484
7. Matsuda M, Shibuya S, Oikawa T, Murakami K, Mochizuki H. A case of late-onset aqueductal membranous occlusion and a successful treatment with neuro-endoscopic surgery. Clin Neurol. 2011. 51: 590-4
8. Schroeder HW, Gaab MR. Endoscopic aqueductoplasty: Technique and results. Neurosurgery. 1999. 45: 508-15
9. Steel T, Maixner WJ, Chaseling R, Johnston I. Demonstration of membranous aqueduct occlusion by fast multiphase magnetic resonance imaging. J Clin Neurosci. 1997. 4: 352-4
10. Terada Y, Yamamoto M, Motoie R, Matsui Y, Katsuki T, Mori N. Hydrocephalus resulting from late-onset aqueductal membranous occlusion: A case report and review of the literature. World Neurosurg. 2020. 137: 345-9
11. Utsuki S, Osano S, Endo M, Mizokami K. A elderly case of late-onset aqueductal membranous occlusion was denied idiopathic normal pressure hydrocephalus by tap test. Neurosurg Cogn Disorder. 2021. 1: 11-6