- Department of Neurosurgery, ASUFC Santa Maria della Misericordia, Piazzale Santa Maria della Misericordia, Udine, Italy.
DOI:10.25259/SNI_519_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Sara Fabbro, Francesco Tuniz, Daniele Piccolo, Antonio Cramaro. Late-onset occlusion of the Monro foramina after endoscopic third ventriculostomy in adults: Case discussion and review of the literature. 08-Oct-2020;11:326
How to cite this URL: Sara Fabbro, Francesco Tuniz, Daniele Piccolo, Antonio Cramaro. Late-onset occlusion of the Monro foramina after endoscopic third ventriculostomy in adults: Case discussion and review of the literature. 08-Oct-2020;11:326. Available from: https://surgicalneurologyint.com/surgicalint-articles/10313/
Abstract
Background: Few cases of adult idiopathic occlusion of the foramen of Monro (AIOFM) are described in the literature. The diagnosis of AIOFM after an endoscopic procedure is even more infrequent.
Case Description: We described the case of a 50-year-old woman who developed bilateral membranous occlusion of both Monro foramina 20 years after an endoscopic third ventriculostomy (ETV) for triventricular hydrocephalus due to an aqueductal stenosis. The patient underwent an endoscopic treatment (left foraminoplasty and septostomy) to check the patency of the stoma on the floor of the third ventricle. After the endoscopic procedure, the symptoms improved and the postoperative magnetic resonance imaging (MRI) demonstrated the resolution of the biventricular hydrocephalus.
Conclusion: Bilateral occlusion of both FM with consequent bilateral lateral ventricles enlargement is an extremely rare condition, especially if we consider the cases of biventricular hydrocephalus after endoscopic procedures. In our opinion, an endoscopic approach should be attempted as first choice procedure, avoiding any intraventricular stent or shunt placement.
Keywords: Endoscopic third ventriculostomy, Foraminoplasty, Hydrocephalus, Idiopathic, Neuroendoscopy, Septostomy
INTRODUCTION
Adult idiopathic occlusion of the foramen of Monro (AIOFM) is an exceeding rare condition, with few cases reported in the literature.[
A recent review has classified the AIOFM into four classes, on the bases of the symmetry of the lateral ventricles enlargement (and, consequently, on the deviation of the septum) and the presence/absence of membranes at the level of the FM.[
In our experience, only four cases of bilateral occlusion of FM after previous endoscopic procedures have been reported in the literature.[
CASE DESCRIPTION
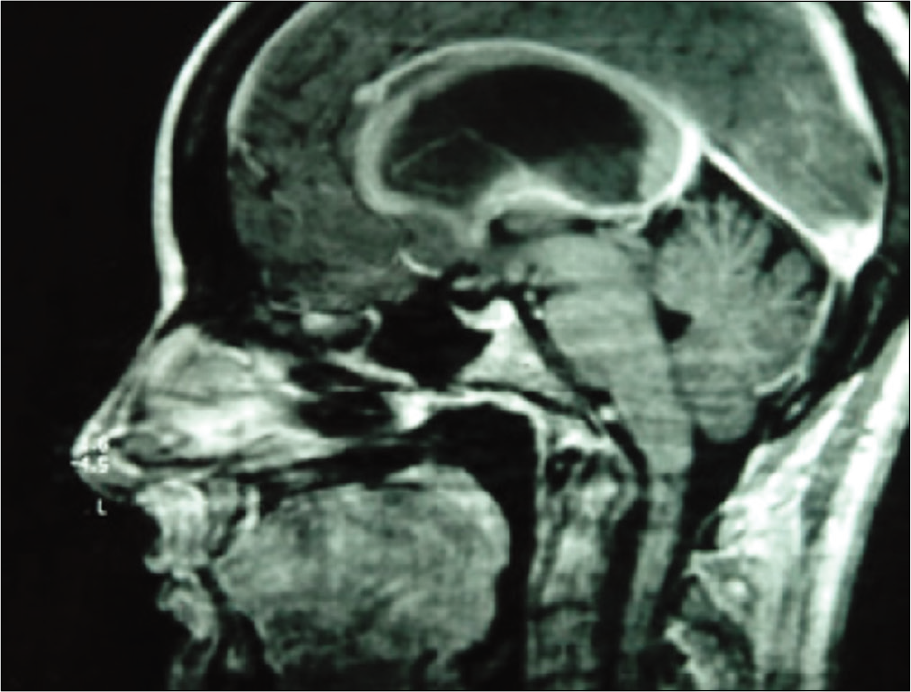
Due to frequent episodes of headache, a 30-year-old female underwent neuroradiological investigations that documented the presence of an aqueductal stenosis with triventricular hydrocephalus and transependymal transudation [
Figure 1:
T1-weighted MRI sequence performed before the endoscopic third ventriculostomy. On this MRI, performed 20 years ago and for this reason of suboptimal quality, we identified the presence of a dilatation of the supratentorial compartment of the ventricular system and the presence of a membranous aqueductal stenosis.
During the procedure, no hemorrhage occurred. Neither symptoms nor signs of infection were identified during the hospitalization and the patient’s symptoms improved. She underwent regular clinical and neuroradiological follow-up. In particular, the MRI demonstrated a decrease in size of the third and the lateral ventricles, with the resolution of the transependymal transudation. The fast spin-echo (FSE) sequences revealed the patency of the stoma and the regular flow void inside it.
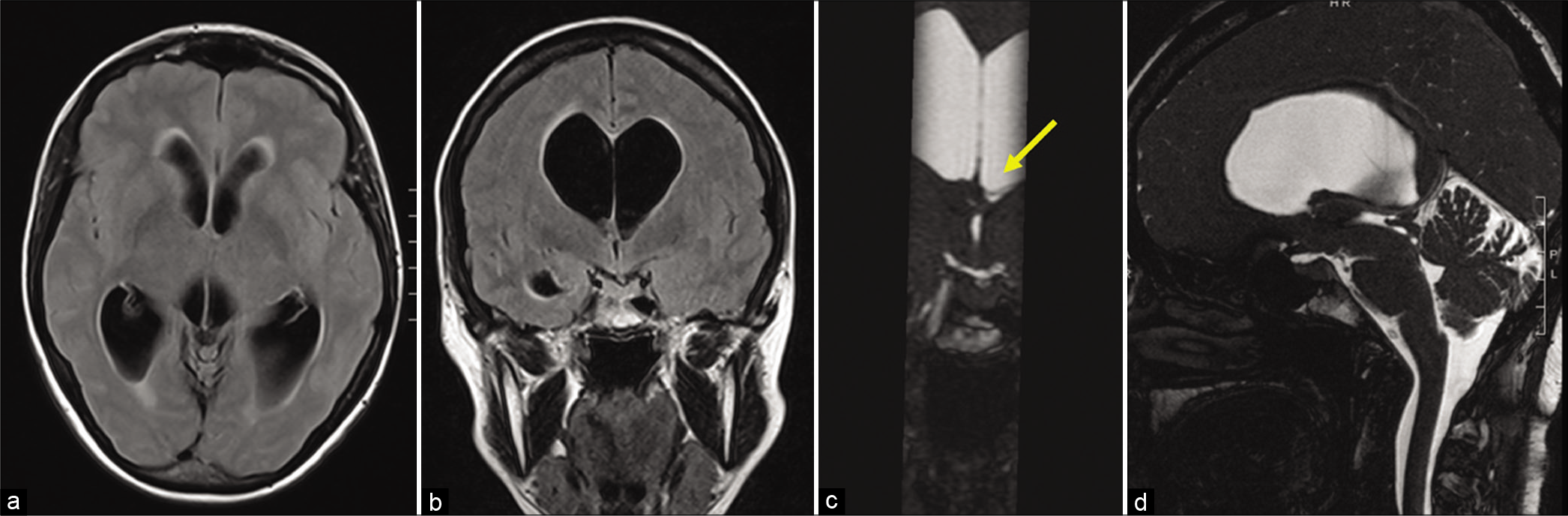
Twenty years after the endoscopic procedure, the patient complained again of severe headaches, acute onset of gait imbalance, dizziness, and vomiting. The MRI showed a dilatation of the lateral ventricles with slight deviation of the septum and slit third ventricle, indicating an obstruction of both FM [
Figure 2:
Preoperative MRI. The preoperative MRI demonstrated a biventricular hydrocephalus with sings of transependymal transudation (a). The coronal scans documented that the right lateral ventricle was more dilated than the left one (b) and both FM were occluded (c), with an apparent enlargement of the left FM aditus (yellow arrow). In the sagittal plane, the stoma was not identifiable, while it was possible to objectify an aqueductal stenosis due to the presence of septa inside it (d).
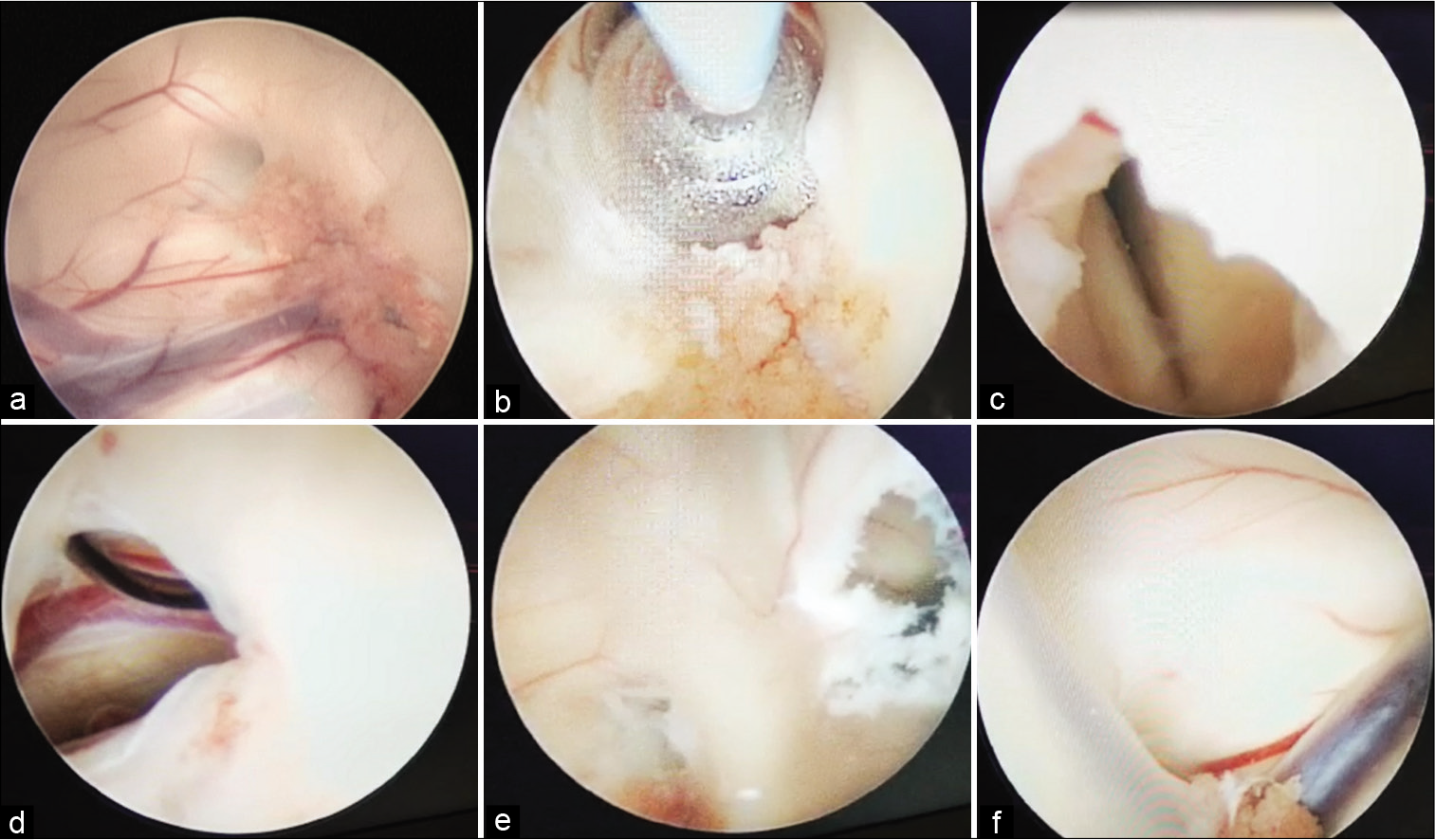
Figure 3:
Intraoperative findings. Performing a left-sided approach, we observed that a thin fibrous membrane occluded the homolateral FM (a). We enlarged the left FM (b) and entered in a slit third ventricle (c). We documented the patency of the endoscopic third ventriculostomy (d). We also perforated the septum (e) and, through the septostomy, we evaluated the right FM (f).
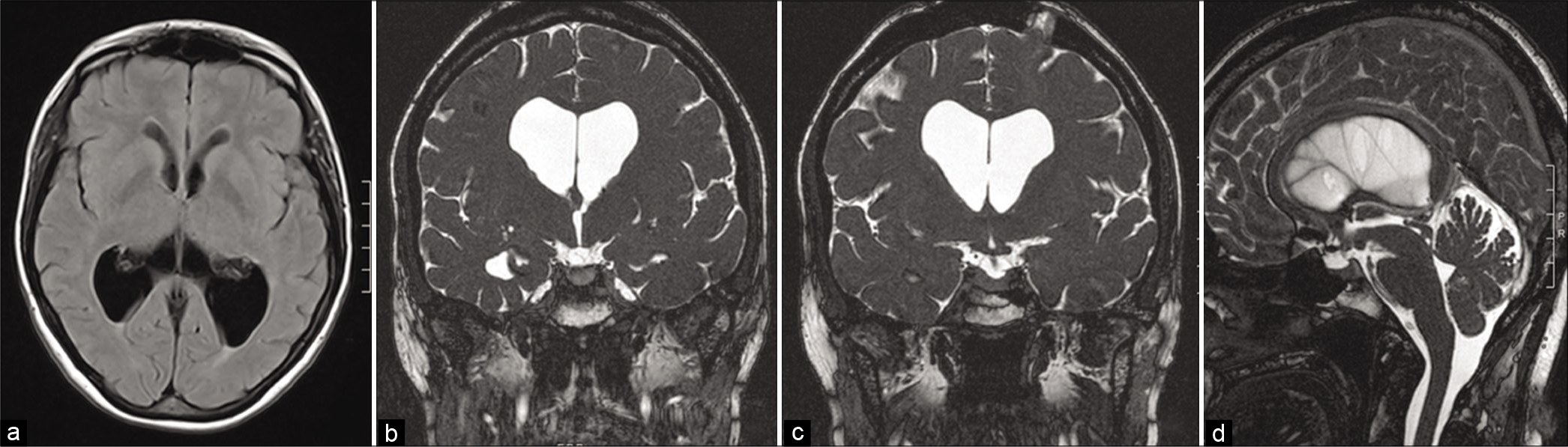
After surgery, the symptoms improved and the early postoperative MRI showed the resolution of the biventricular hydrocephalus and the patency of septostomy, foraminoplasty, and ETV [
Figure 5:
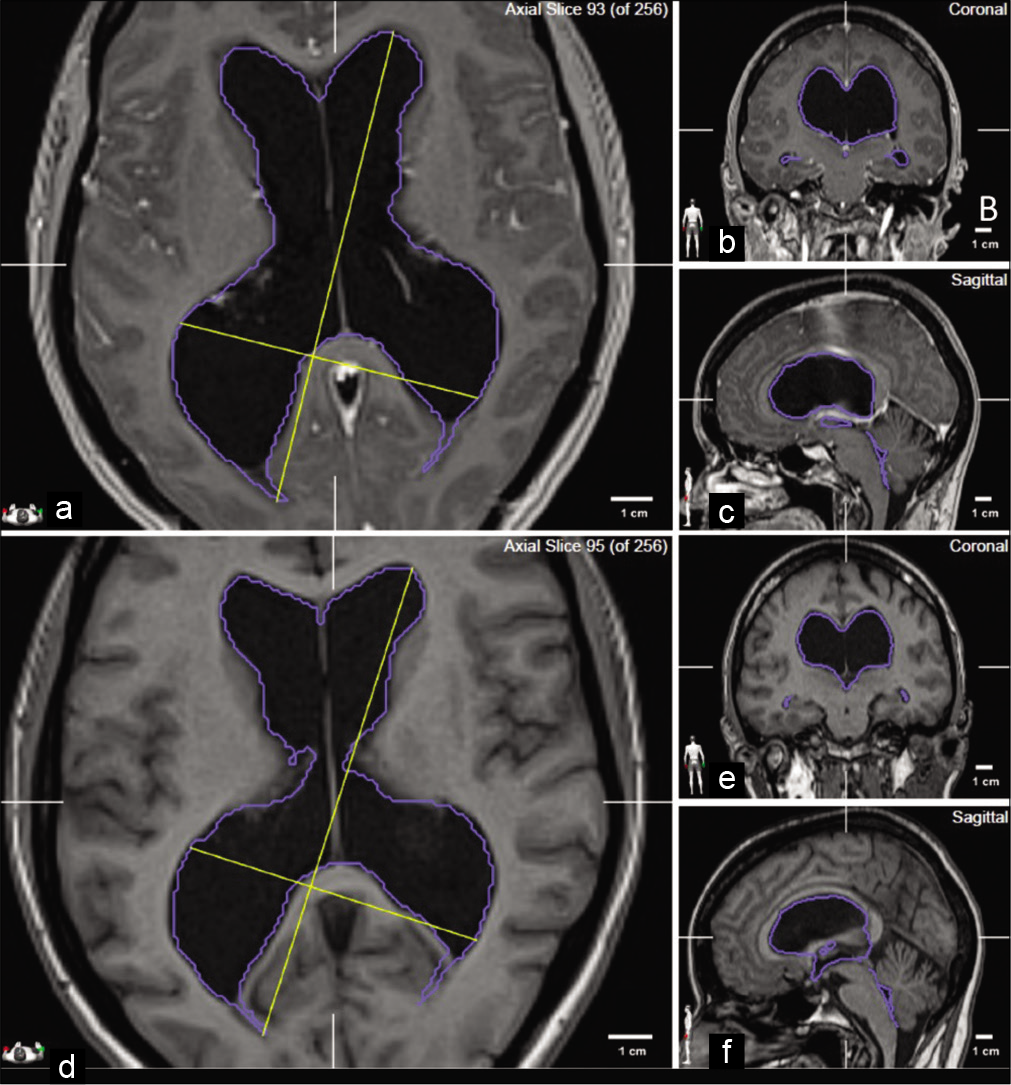
Volumetric analysis. To objectify the reduction of the ventricular system dilatation, we segmented the preoperative (a-c) and postoperative MRI (d-f). Comparing axial (a,d), coronal (b,e), and sagittal (c,f) images, we identified a reduction of the biplanar dimensions of the ventricular system. This reduction was confirmed by the analysis of the preoperative (191.8 cm3) and postoperative (135.9 cm3) volumes.
A year later, the patient remained asymptomatic and no changes on the cerebral MRI were observed.
DISCUSSION
AIOFM is a rare condition without a clear etiology or a standardized treatment.[
The first report is that of Marions, in 1986, who described two cases of true bilateral stenosis of the FM in the pre-MRI era. One patient was treated microsurgically, and in both cases, a ventriculoperitoneal shunt (VPS) was implanted.[
In 1992, Maxwell reported the case of a female with striking rapid memory loss and documented a type 1 AIOFM (sec. Ebrahimzadeh[
The first case of AIOFM treated endoscopically was reported in 2001 by Javier-Fernández who performed a foraminoplasty using a bipolar cauterization without septostomy.[
Some observations have been made on the imaging data and the modality to perform the stomas. In particular, while in some cases, the diagnosis was reached using ventriculography[
Relatively to the instrumentation, Schonauer concluded that the use of the thulium laser, instead of the Fogarty balloon, could reduce the risk of unintended violation of the fornix or of the other neural structures.[
While there are some cases of AIOFM treated endoscopically,[
A similar case was reported by Migliorati: he described the case of a 52-year-old woman with bilateral occlusion of both foramina, who was initially treated with VPS insertion. Because of the onset of an abdominal infection, the shunt was removed and the bilateral dilatation of both lateral ventricles was treated with endoscopic foraminoplasty and septostomy. Rapidly, the hydrocephalus recurred and the patient underwent a ventriculoatrial (VA) shunt insertion resulting in resolution of her symptoms.[
El Rafee presented the case of a 30-year-old female who underwent ETV for occlusive hydrocephalus due to aqueductal stenosis. After 30 months, she reported recurrent headaches and the MRI demonstrated bilateral enlargement of the lateral ventricles with a collapsed third ventricle caused by bilateral stenosis of the FM. A left-sided endoscopic foraminoplasty and stenting of the left FM were performed with immediate neurological improvement. The authors ascribed the bilateral occlusion of the FM to the small diameters of these foramina before the first surgery: after the ETV, the diminished size of the lateral ventricles induced a decreasing in the FM diameter and the voluminous choroid plexus blocked the CSF flow within the foramina.[
Nagata reported the case of a 10-year-old girl with triventricular hydrocephalus due to aqueductal stenosis. She underwent ETV and reported no symptoms for 32 months. After that period, the patient presented with an exacerbation of headaches and the MRI findings demonstrated the bilateral occlusion of FM with a slim third ventricle. She underwent a septostomy (with monopolar microendoscopic electrode) and VPS insertion. The authors could not exclude the possibility of slight mechanical injury during the first endoscopic procedure nor of a subclinical postoperative intraventricular hemorrhage or of a ventriculitis. Because of the small dimension of the choroid plexus and consequent little production of CSF, Nagata hypothesized that the stagnation of the CSF could contribute to the occlusion of the FM.[
Similar to our experience, as in the majority of these cases, no sign of infection nor hemorrhage was reported.[
Not relying only on macroscopic findings, we could not exclude the role of cellular abnormalities in the occlusion of both FM. While some authors focused their attention on ependymal cells[
Regarding the technique, we used a rigid endoscope obtaining a good visualization of the third ventricle and both lateral ventricles (through the septostomy). Mori reported a more maneuverability of the fiberscope than rigid endoscope inside the ventricular system.[
We performed the left foraminoplasty, the septostomy using a laser coagulator, similarly to Schonauer[
Despite Mori[
CONCLUSION
Bilateral occlusion of FM after endoscopic procedures is an extremely rare condition with no clear etiology nor encoded treatment.
In our opinion, the endoscopic approach should be attempted as the first choice procedure without any intraventricular stent or shunt placement. The use of the intraoperative laser could be of aid to avoid any damage of the internal veins during the foraminoplasty and the septostomy.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Abderrahmen K, Aoudij ML, Kallel J, Zammel I, Khaldi MM. Hydrocephalus due to non-tumoral stenosis of foramens of Monro: Report of four cases. Neurochirurgie. 2008. 54: 72-8
2. Abdi K, Lai CH, Paez-Gonzalez P, Lay M, Pyun J, Kuo CT. Uncovering inherent cellular plasticity of multiciliated ependyma leading to ventricular wall transformation and hydrocephalus. Nat Commun. 2019. 9: 1655
3. Azab WA, Alsheikh TM, Elmansoury TM, Abdelnabi EA. Endoscopic treatment of adult idiopathic obstruction of the foramen of Monro. Acta Neurochir (Wien). 2017. 159: 1439-43
4. Banizs B, Pike MM, Millican CL, Ferguson WB, Komlosi P, Sheetz J. Dysfunctional cilia lead to altered ependyma and choroid plexus function, and result in the formation of hydrocephalus. Development. 2005. 132: 5329-39
5. de Bonis P, Anile C, Tamburrini G, Tartaglione T, Mangiola A. Adult idiopathic occlusion of the foramina of Monro: Diagnostic tools and therapy. J Neuroimaging. 2008. 18: 101-4
6. Ebrahimzadeh K, Maloumeh EN, Samadian M, Rezaei O. Endoscopic strategy in surgical treatment of adult idiopathic bilateral occlusion of the foramen of Monro and review of the literature. World Neurosurg. 2018. 115: e610-9
7. El Rafee E, Baldauf J, Schroeder HW. Bilateral occlusion of the foramina of Monro after third ventriculostomy. J Neurosurg. 2012. 116: 1333-6
8. Freudenstein D, Duffner F, Krapf H, Wagner A, Grote EH. Neuroendoscopic treatment of idiopathic occlusion of the foramen of Monro in adults-two case reports. Neurol Med Chir (Tokyo). 2002. 42: 81-5
9. Javier-Fernández J, Garcia-Cosamalon PJ, Vinuela J, Ibañez FJ, Mostaza A, Heres S. Endoscopic fenestration as a treatment for asymmetrical hydrocephalus due to obstruction of the foramen of Monro. Neurocirugia (Astur). 2001. 12: 513-5
10. Kalhorn SP, Strom RG, Harter DH. Idiopathic bilateral stenosis of the foramina of Monro treated using endoscopic foraminoplasty and septostomy. Neurosurg Focus. 2011. 30: E5
11. Maldonado IL, de Champfleur NM, Bonafé A, El-Fertit H. Bilateral idiopathic occlusion of the foramina of Monro. Eur Neurol. 2012. 67: 167
12. Marions O, Boethius J. Congenital constriction of the foramen Monro. Neuroradiology. 1986. 28: 275-8
13. Martinez-Berganza MT, Bergua BS, del Rio Perez C, Ballarìn S. Biventricular hydrocephalus due to idiopatic occlussion of foramina of Monro. Neurologist. 2011. 17: 154-6
14. Maxwell JA, Stimac GK. Adult “congenital” bilateral occlusion of the foramina of Monro. Surg Neurol. 1992. 37: 51-3
15. Migliorati K, Muratori A, Fontanella MM, Panciani PP. Adult bilateral idiopathic occlusion of foramina of Monro: Is foraminoplasty really safe and effective?. BMJ Case Rep. 2018. 2018: bcr2018226332
16. Mizrahi CJ, Cohen JE, Gomori JM, Shoshan Y, Spektor S, Moscovici S. Idiopathic bilateral occlusion of the foramen of Monro: An unusual entity with varied clinical presentations. J Clin Neurosci. 2016. 34: 140-4
17. Mori H, Koike T, Fujimoto T, Nishiyama K, Yoshimura J, Tanaka R. Endoscopic stent placement for treatment of secondary bilateral occlusion of the Monro foramina following endoscopic third ventriculostomy in a patient with aqueductal stenosis. Case report. J Neurosurg. 2007. 107: 416-20
18. Nagata Y, Takeuchi K, Nagatani T, Watanabe T, Sato Y, Tambara M. Bilateral occlusion of the foramina of Monro after endoscopic third ventriculostomy for aqueductal stenosis-a case report. Childs Nerv Syst. 2016. 32: 739-43
19. Narita K, Takeda S. Cilia in the choroid plexus: Their roles in hydrocephalus and beyond. Front Cell Neurosci. 2015. 9: 39
20. Ohata S, Álvarez-Buylla A. Planar organization of multiciliated ependymal (E1) cells in the brain ventricular epithelium. Trends Neurosci. 2016. 39: 543-51
21. Olstad EW, Ringers C, Hansen JN, Wens A, Brandt C, Wachten D. Ciliary beating compartmentalizes cerebrospinal fluid flow in the brain and regulates ventricular development. Curr Biol. 2019. 29: 229-41
22. Prontera A, Feletti A, Chahine R, Pavesi G. Adult idiopathic occlusion of Monro foramina: Intraoperative endoscopic reinterpretation of radiological data and review of the literature. Br J Neurosurg. 2015. 29: 609-10
23. Raz E, Fatterpekar G, Davis AJ, Huang PP, Loh JP. Mystery case: Idiopathic bilateral stenosis of the foramina of Monro. Neurology. 2012. 79: 166-7
24. Schonauer C, Johnson R, Chiratti S, de Falco R, Albanese V, Tessitore E. Adult idiopathic occlusion of Monro foramina: Intraoperative endoscopic reinterpretation of radiological data and review of the Literature. Br J Neurosurg. 2014. 28: 717-21
25. Sharifi G, Alavi E, Rezaee O, Jahanbakhshi A, Faramarzi F. Neuroendoscopic foraminoplasty for bilateral idiopathic occlusion of foramina of Monro. Turk Neurosurg. 2012. 22: 265-8
26. Wong TT, Lee LS. Membranous occlusion of the foramen of Monro following ventriculoperitoneal shunt insertion: A role for endoscopic foraminoplasty. Childs Nerv Syst. 2000. 16: 213-7