- Department of Neurosurgery and Spine Surgery, Hanoi Medical University Hospital, Hanoi, Vietnam.
- Department of Pathology, Hanoi Medical University Hospital, Hanoi, Vietnam.
Correspondence Address:
Tam Duc Le, Department of Neurosurgery and Spine Surgery, Hanoi Medical University Hospital, Hanoi, Vietnam.
DOI:10.25259/SNI_663_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Hung Dinh Kieu1, Duong Van Dang2, Tam Duc Le1. Late recurrence of primary cerebellar germinoma at unusual site after complete response to radiotherapy. 02-Nov-2021;12:549
How to cite this URL: Hung Dinh Kieu1, Duong Van Dang2, Tam Duc Le1. Late recurrence of primary cerebellar germinoma at unusual site after complete response to radiotherapy. 02-Nov-2021;12:549. Available from: https://surgicalneurologyint.com/surgicalint-articles/11207/
Abstract
Background: The primary cerebellar germinoma is exceptional and difficult to diagnose preoperatively. Its recurrence at the middle cranial fossa after complete response to radiotherapy is unique and associated with a poor prognosis. This article aims to report the successful management of the late recurrence of primary cerebellar germinoma at an unusual site after 4 years of complete response to radiotherapy.
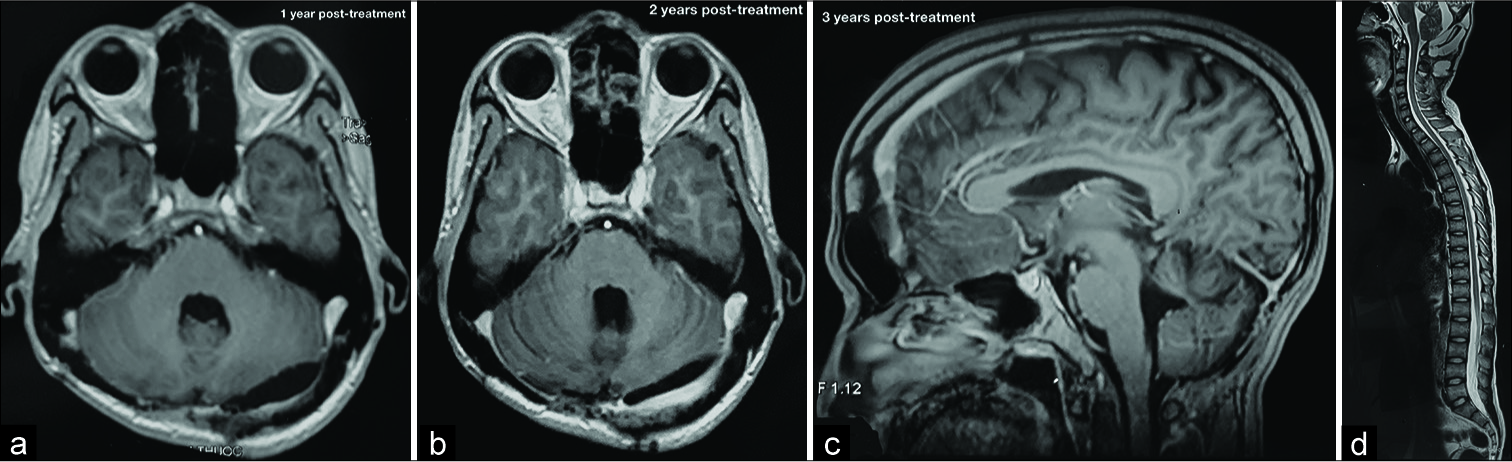
Case Description: A 22-year-old male was admitted to our hospital with complaints of severe headache and loss of balance. Brain magnetic resonance imaging (MRI) showed a triventricular hydrocephalus due to a 45x50mm cerebellar mass. Our preliminary diagnosis was medulloblastoma. First, we placed a ventriculoperitoneal shunt with the medium-pressure valve, and then we used midline suboccipital craniotomy to remove the tumor completely. The histopathology was germinoma. The patient received 24 Gy craniospinal irradiation (CSI) with a 16 Gy boost to the primary site and had an MRI follow-up every six months. After a 4-year follow-up, he complained of recurrent severe headaches. The brain MRI illustrated a 62 × 61 mm temporal mass. We extirpated this tumor, and histopathology again revealed germinoma. After that, the patient received induction radiotherapy. The 1-year postoperative MRI showed no tumor remnant. At the time of writing, the patient had no headache and no neurological deficits.
Conclusion: Regular follow-ups with routine neuroaxis MRI should be recommended to detect recurrence early for all patients with intracranial germinomas. Surgical resection, if possible, and subsequent CSI are the most effective salvage treatment for recurrent germinoma.
Keywords: Craniospinal irradiation, Late recurrence, Primary cerebellar germinoma, Radiotherapy
INTRODUCTION
Intracranial pure germinoma is the most common neoplasm (70-80%) of germ cell tumors derived from germ cells.[
Pure germinoma is radiosensitive; thus, radiotherapy is recommended as the first-line therapy with 5-year progression-free survival and overall survival rates of more than 90%.[
Despite high radiosensitivity, the proportion of recurrent germinoma after complete response to radiotherapy is estimated to be 6–17%.[
This article aims to report the successful management of the late recurrence of primary cerebellar germinoma at an unusual site after four years of complete response to radiotherapy.
PRESENTATION OF CASE
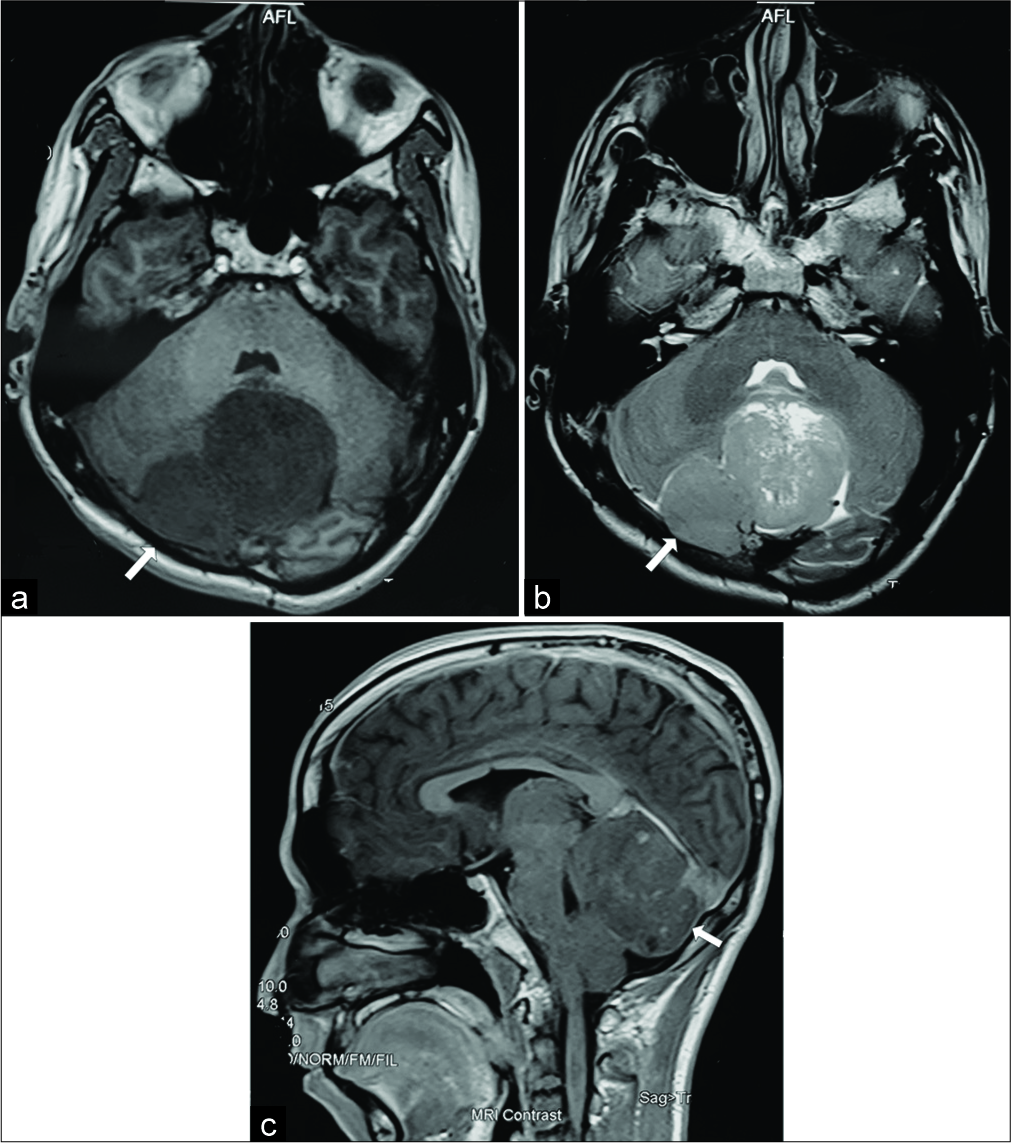
A 22-year-old male was admitted to our hospital with complaints of severe headache and loss of balance. On examination, he was alert and oriented. He had cerebellar ataxia and imbalance. The motor and sensory functions of the extremities were normal. He denied cranial nerve palsies. Brain magnetic resonance imaging (MRI) showed a cerebellar mass measuring 45x50 millimeters [
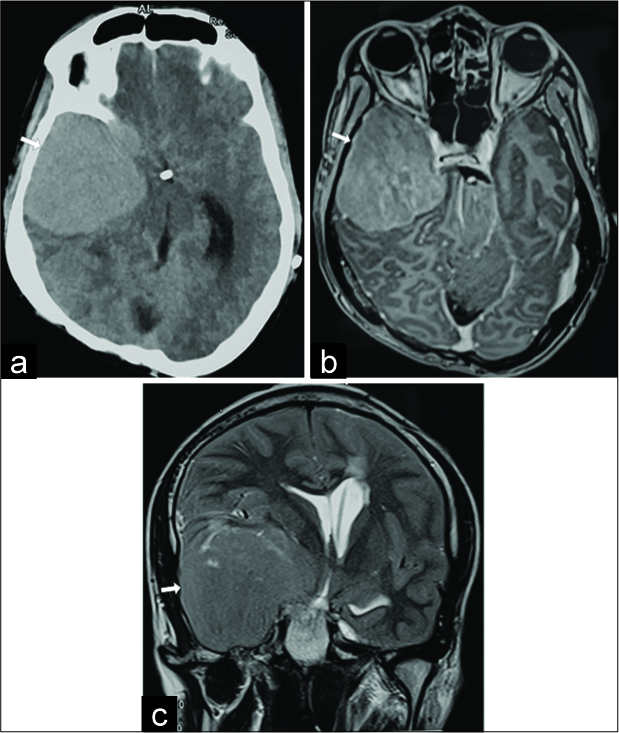
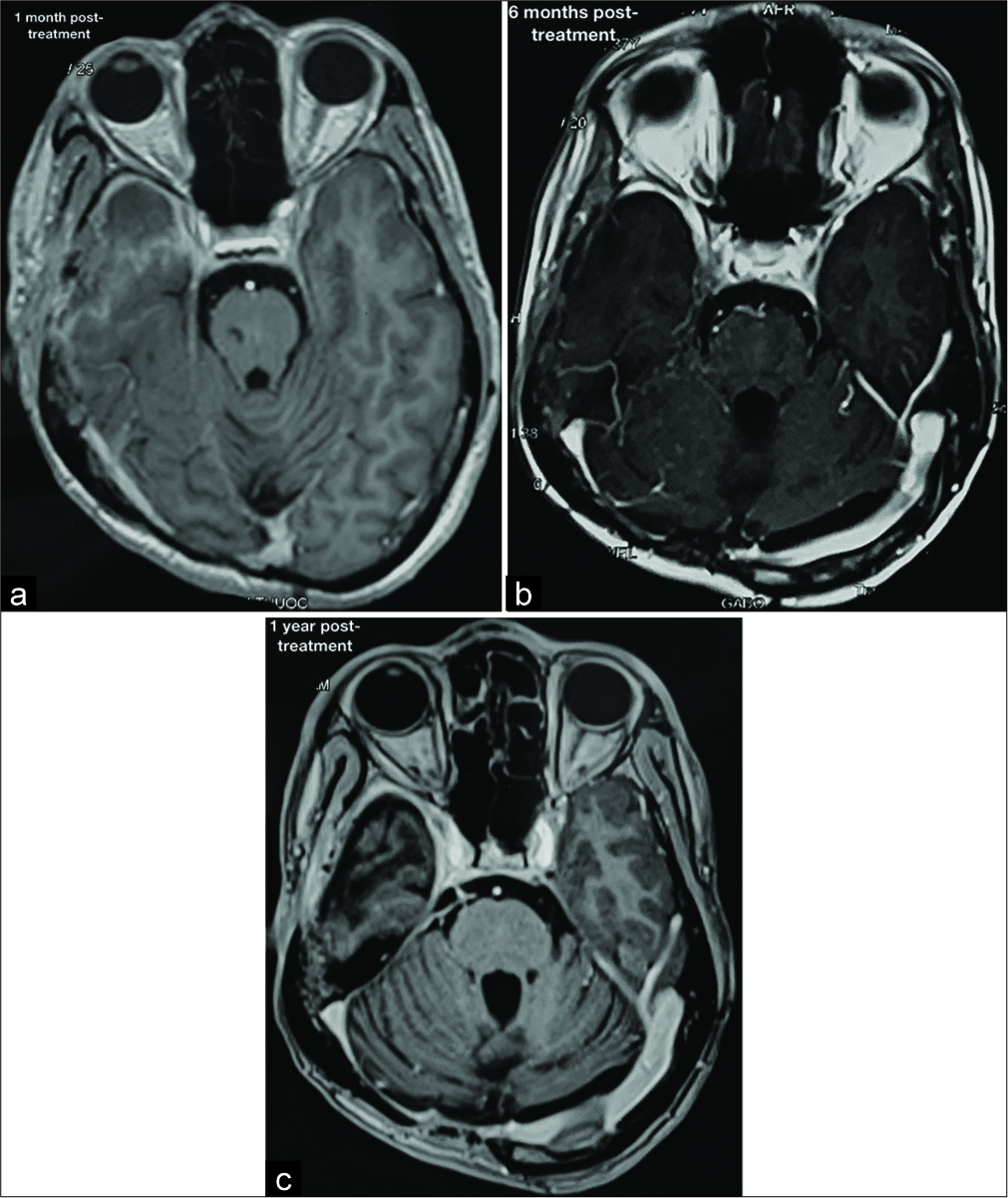
After a 4-year follow-up, when he was 26 years old, he was admitted again to our hospital with complaints of recurrent severe headache. The physical examination revealed no neurological deficits. The brain MRI illustrated a temporal mass measuring 62 × 61 mm. The temporal mass was hyperdense on computed tomography, isointense on T1W sequence, hyperintense on T2W sequence, and enhanced heterogeneously on T1W postgadolinium [
DISCUSSION
The primary cerebellar germinoma of the posterior fossa is unique. Only six cases were reported in previous studies, in which only three patients had isolated cerebellar germinoma, as was the case of our patient.[
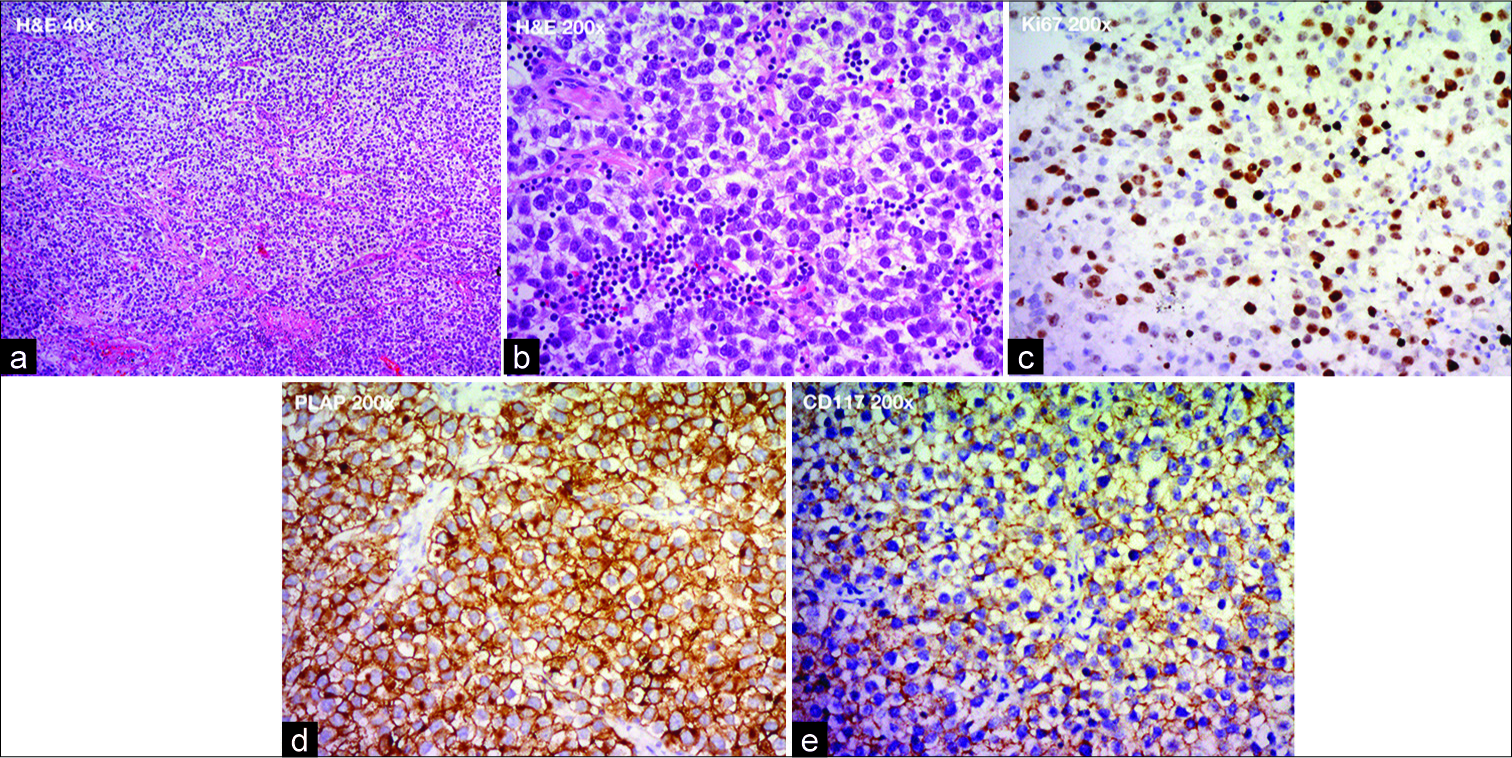
Furthermore, the common sites of recurrence are the ventricle and spine, whereas our case had a late recurrence at the middle cranial fossa, an unusual location. Thus, the preoperative diagnosis of recurrent germinoma at the middle cranial fossa was more challenging. In this case, surgery and histopathological assessment were essential for a definitive diagnosis. Besides, immunohistochemical staining may be helpful to rule out differential diagnoses in patients with unusual localizations and untypical histological features.[
Despite high radiosensitivity, the proportion of recurrent germinoma after complete response to radiotherapy is estimated to be 6–17%.[
Salvage treatments for recurrent intracranial germinoma vary among previous studies. More than half (58.8%) of patients received salvage radiotherapy and chemotherapy for recurrent germinoma. In which CSI was the most preferred choice of radiotherapy.[
Regarding chemotherapy for germinoma, chemotherapy alone cannot be used due to its due to dismal survival rates (5-year PFS <50%).[
Regarding prognostic factors, age at diagnosis, gender, primary site, time to recurrence, site of recurrence, and patterns of failure were not statistically significant on univariate analysis. On multivariate analysis, Hu et al. demonstrated that salvage radiotherapy and initial radiotherapy doses were significant predictors of better survival time after recurrence. Interestingly, patients who received initial radiotherapy with intermediate doses had better outcomes than those with high doses (P = 0.049).[
CONCLUSION
The primary cerebellar germinoma is exceptional and difficult to diagnose preoperatively. Its recurrence after a complete response to radiotherapy is unique. Surgical resection, if possible, and adjuvant CSI are the most effective salvage treatment for recurrent germinoma. Regular surveillance follow-ups with routine neuroaxis MRI should be recommended to detect recurrence early for all patients with intracranial germinomas.
Ethical approval
The study was approved by the Research Ethics Committee of Hanoi Medical University. The procedures used in this study adhere to the tenets of the Declarations of Helsinki.
Research registry
Not applicable – this is a single case report, not a systematic review or meta-analysis. Moreover, we attest that it is not a ‘first in man’ study, either.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Al-Hussaini M, Sultan I, Abuirmileh N, Jaradat I, Qaddoumi I. Pineal gland tumors: Experience from the SEER database. J Neurooncol. 2009. 94: 351-8
2. Blakeley JO, Grossman SA. Management of pineal region tumors. Curr Treat Options Oncol. 2006. 7: 505-16
3. Calaminus G, Kortmann R, Worch J, Nicholson JC, Alapetite C, Garrè ML. SIOP CNS GCT 96: Final report of outcome of a prospective, multinational nonrandomized trial for children and adults with intracranial germinoma, comparing craniospinal irradiation alone with chemotherapy followed by focal primary site irradiation for patients with localized disease. Neuro Oncol. 2013. 15: 788-96
4. da Silva NS, Cappellano AM, Diez B, Cavalheiro S, Gardner S, Wisoff J. Primary chemotherapy for intracranial germ cell tumors: Results of the third international CNS germ cell tumor study. Pediatr Blood Cancer. 2010. 54: 377-83
5. Eom KY, Kim IH, Park CI, Kim HJ, Kim JH, Kim K. Upfront chemotherapy and involved-field radiotherapy results in more relapses than extended radiotherapy for intracranial germinomas: Modification in radiotherapy volume might be needed. Int J Radiat Oncol Biol Phys. 2008. 71: 667-71
6. Hu YW, Huang PI, Wong TT, Ho DM, Chang KP, Guo WY. Salvage treatment for recurrent intracranial germinoma after reduced-volume radiotherapy: A single-institution experience and review of the literature. Int J Radiat Oncol Biol Phys. 2012. 84: 639-47
7. Kahn L, Fridley J, Patel AJ, Gressot L, Kitagawa R, Goodman JC. Disseminated germinoma in the brain and cervical spinal cord 10 years after radiographic resolution of pineal germinoma. J Clin Neurosci. 2012. 19: 1055-7
8. Louis DN, Perry A, Reifenberger G, von Deimling A, FigarellaBranger D, Cavenee WK. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016. 131: 803-20
9. Maiuri F, Cappabianca P, de Caro MB, Esposito F, de Divitiis E. Primary cerebellar germinomas of the posterior fossa. Br J Neurosurg. 2004. 18: 284-9
10. Matsutani M, Sano K, Takakura K, Fujimaki T, Nakamura O, Funata N. Primary intracranial germ cell tumors: A clinical analysis of 153 histologically verified cases. J Neurosurg. 1997. 86: 446-55
11. Ono N, Isobe I, Uki J, Kurihara H, Shimizu T, Kohno K. Recurrence of primary intracranial germinomas after complete response with radiotherapy: Recurrence patterns and therapy. Neurosurgery. 1994. 35: 615-21
12. Shima H, Nishizaki T, Ishihara H, Moroi J, Fujii M, Suzuki M. Recurrent intracranial germinoma with dissemination along the ventricular catheter: A case report. J Clin Neurosci. 2002. 9: 708-10
13. Wang WG, Ye H, Chinnaiyan P. Practice patterns and survival outcomes of intracranial germinoma: An analysis of the national cancer database. J Neurooncol. 2018. 137: 77-82