- Department of Surgery, Division of Neurosurgery, University of Toledo, Toledo, United States.
Correspondence Address:
Andrew Leland Waack, Department of Surgery, Division of Neurosurgery, University of Toledo, Toledo, United States.
DOI:10.25259/SNI_151_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Waack AL1, Sharkey B1, Hoyt A1, Schroeder JL1. Letter to the Editor regarding “Clear-cell renal cell carcinoma and glioblastoma multiforme coexistence: Double primary malignancy, does it have a causal relationship?”. Surg Neurol Int 07-Apr-2023;14:134
How to cite this URL: Waack AL1, Sharkey B1, Hoyt A1, Schroeder JL1. Letter to the Editor regarding “Clear-cell renal cell carcinoma and glioblastoma multiforme coexistence: Double primary malignancy, does it have a causal relationship?”. Surg Neurol Int 07-Apr-2023;14:134. Available from: https://surgicalneurologyint.com/surgicalint-articles/12235/
Dear Editor,
We recently read with great interest “Clear-cell renal cell carcinoma (RCC) and glioblastoma (GBM) multiforme coexistence: Double primary malignancy, does it have a causal relationship?” by Simanjuntak et al., who describes a case of concurrent RCC and GBM.[
CASE DESCRIPTION
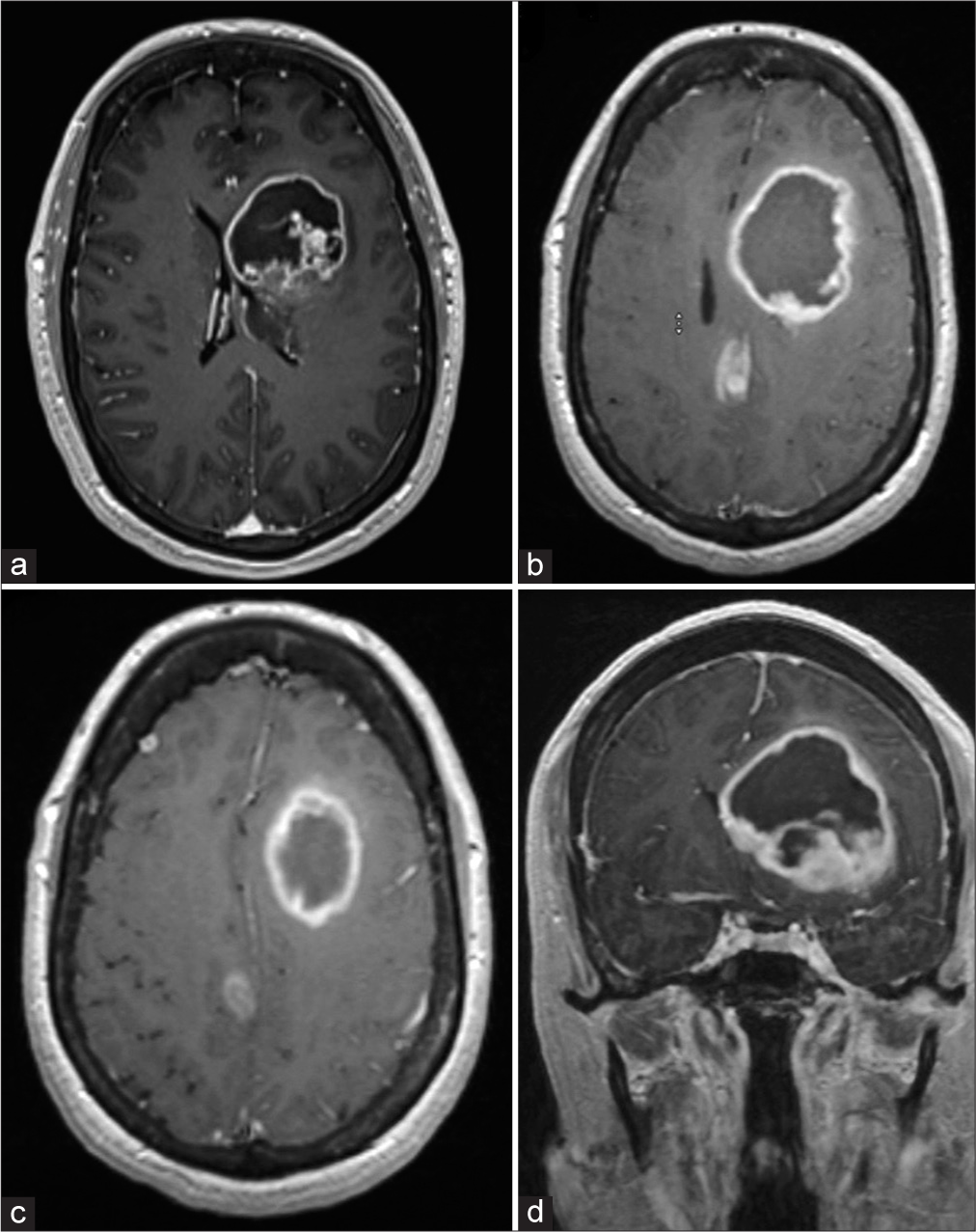
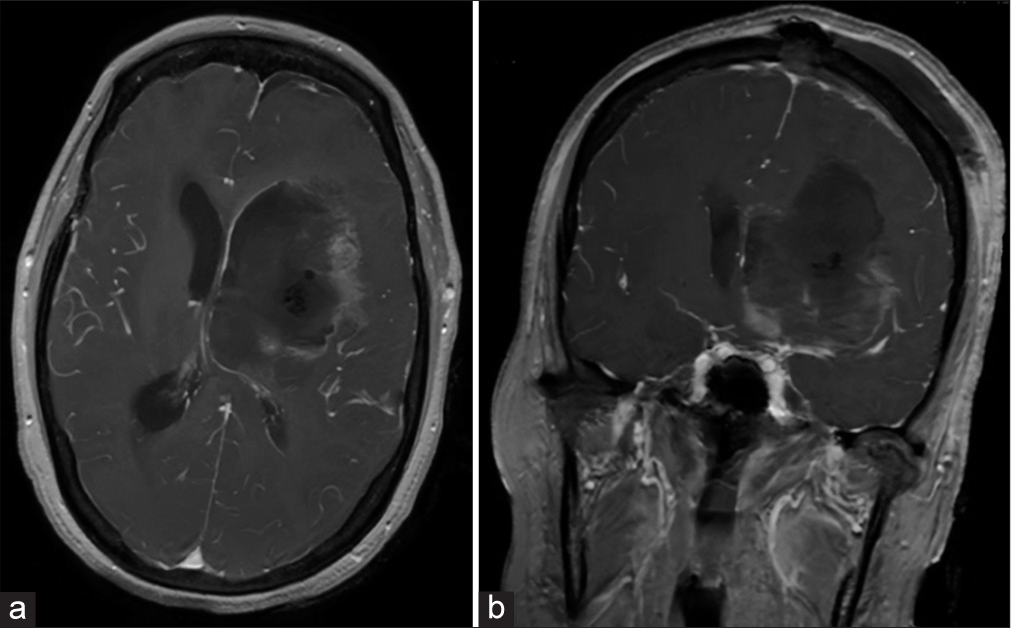
A 61-year-old previously high functioning woman presented with word finding difficulty, memory changes, and difficulty drawing shapes. Prior medical history included RCC treated with nephrectomy 4 years prior. Subsequent magnetic resonance imaging (MRI) revealed a 6.3 × 5.6 × 5.5 cm deep left frontal lesion, with an additional right frontal juxtacortical lesion (0.5 cm maximal diameter), and a third lesion in the right cingulate gyrus (2 cm maximal diameter); all three lesions demonstrated peripheral enhancement [
Figure 1:
(a) Axial T1-weighted contrast-enhanced magnetic resonance (MR) image obtained 3 weeks before surgery. The lesion measures 4.2 × 4.5 cm. (b and c). Axial T1-weighted contrast enhanced MR image obtained 3 weeks after the MRI in (a). The tumor measured grew to 6.3 × 5.5 cm. All three tumors are visible in (c and d). Coronal T1-weighted contrast-enhanced MR image obtained 3 weeks after the magnetic resonance imaging in (a).
LITERATURE REVIEW
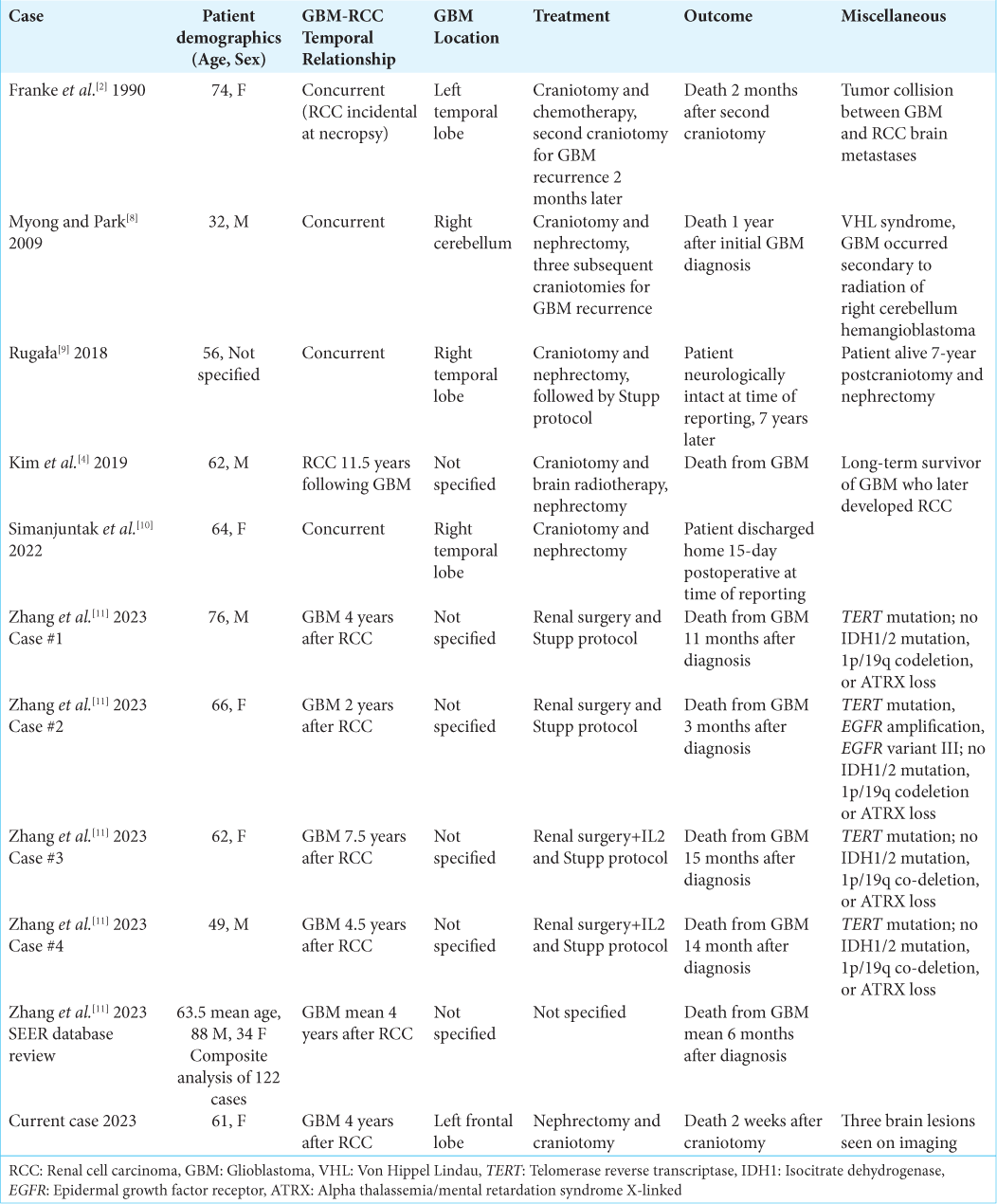
According to our literature search, our case represents the 132nd known patient with both RCC and GBM. Simanjuntak et al. have admirably described both their case and Franke et al.’s cases; we refer readers to review Simanjuntak et al. for a complete description of these cases.[
Rugala describes a 56-year-old female who presented for new onset seizures, with subsequent MRI demonstrating a T2 hyperintense lesion with surrounding edema in the right temporal lobe; abdominal computed tomography demonstrated a heterogeneous hypodense lesion in the right kidney. A subsequent craniotomy was performed, followed shortly soon after by nephrectomy. Brain histopathological analysis revealed a neoplasm of glial origin, with immunohistochemistry demonstrating: casein kinase 1 (CK 1) (−), glial fibrillary acidic protein (GFAP) (+), subsequently diagnosed as GBM; the kidney tumor was diagnosed as RCC. Brain radiation and temozolomide were soon initiated. Amazingly, at the time of publication, 7 years later, the patient was reported to be neurologically intact, with follow-up imaging demonstrating only demyelinating changes in the surgical and radiation site; additionally, there was no proof either of relapse or dissemination of RCC.[ Myong and Park describe the case of a malignant glioma in a patient with Von Hippel-Lindau (VHL) syndrome with RCC and a history of multiple hemangioblastomas. At the age of 25, the patient underwent excision and subsequent radiation therapy for a large right cerebellar hemangioblastoma. The excised lesion stained positive for S100 and negative for p53 and GFAP, findings consistent with hemangioblastoma. Seven years later, the patient presented again for headache and dizziness, with subsequent MRI demonstrating a mass in the prior surgical site with irregular peripheral enhancement and a necrotic core. Abdominal MRI revealed a low-attenuating mass in the right kidney. Craniotomy and nephrectomy were performed soon after. The cranial tumor displayed markedly different morphology and staining patterns compared to the initial hemangioblastoma 7 years prior. This lesion was positive for GFAP and P53 staining and loss of p16, suggesting GBM. The patient had a rapid tumor recurrence and then underwent several episodes of excision and recurrence; the patient ultimately passed away 1 year later.[ Zhang et al. report a series of four cases of metachronous RCC and GBM, as well as an analysis of the SEERS database, identifying 122 cases of RCC followed by GBM. Similar to our reported case, the average GBM occurred approximately 4 years after the diagnosis of RCC. The four GBMs Zhang et al. describe in the case series all had a TERT gene promoter mutation; none of the patients demonstrated IDH1/2 mutation, 1p/19q codeletion, or ATRX loss. Two of the four had TP53 mutations. In the analysis, patients with GBM following RCC had a worse prognosis compared to “control” GBM; Zhang et al. hypothesize the average higher age of GBM and the molecular profiles of these tumors (TERT positive) are likely responsible for the worse outcomes.[ Kim et al. report a case series of malignancies occurring in LTS of GBM. They describe 155 LTS of GBM, with 17 subsequent cancers occurring in 13 patients; most later cancers occurred in the field of radiation for GBM. These cancer types included skin cancer, leukemia, low-grade glioma, and scalp sarcoma. The remaining subsequent cancers reported included melanoma, prostate cancer, bladder cancer, endometrial adenocarcinoma, basal cell carcinoma, and one RCC. The reported RCC occurred 12 years after the initial GBM; this patient’s GBM was managed by gross total resection and radiotherapy, but not temozolomide. The authors did not elaborate on potential mechanisms of RCC induction or the relationship between RCC and GBM.[
COMMENTS
There is no established relationship linking RCC and GBM. Although uncommon, these cancers are not exclusive to one another, and both can occur in the same patient.[
GBM and RCC in the same patient may occur metachronously, with the second malignancy possibly occurring due to therapy for the first malignancy.[
It has been suggested RCC and GBM may occur in the same patient as part of genetic syndromes, although there is no clear evidence as to which genes, alleles, or mutations are responsible.[
There are currently no concrete data demonstrating an association between RCC and GBM, and we agree with Simanjuntak et al. that concurrent RCC and GBM likely represents an unfortunate coincidence, although it is possible, there is an underlying relationship between the two. The literature does not indicate that any specific treatment protocol is particularly effective for these patients. Because there is no known underlying mechanism responsible for the cooccurrence of these cancers, we believe that each cancer should be treated as its own entity, in accordance with the respective standards of care.
We agree with Simanjuntak et al. that it is important to document these unusual cases. RCC and GBM in the same patient are likely to be underdiagnosed and underreported.[
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Bougeard G, Brugieres L, Chompret A, Gesta P, Charbonnier F, Valent A. Screening for TP53 rearrangements in families with the Li-Fraumeni syndrome reveals a complete deletion of the TP53 gene. Oncogene. 2003. 22: 840-6
2. Franke FE, Altmannsberger M, Schachenmayr W. Metastasis of renal carcinoma colliding with glioblastoma. Carcinoma to glioma: An event only rarely detected. Acta Neuropathol. 1990. 80: 448-52
3. Gutmann DH, Rasmussen SA, Wolkenstein P, MacCollin MM, Guha A, Inskip PD. Gliomas presenting after age 10 in individuals with neurofibromatosis Type 1 (NF1). Neurology. 2002. 59: 759-61
4. Kim JY, Jackman JG, Woodring S, McSherry F, Herndon JE, Desjardins A. Second primary cancers in long-term survivors of glioblastoma. Neurooncol Pract. 2019. 6: 386-91
5. Krieg M, Marti HH, Plate KH. Coexpression of erythropoietin and vascular endothelial growth factor in nervous system tumors associated with von Hippel-Lindau tumor suppressor gene loss of function. Blood. 1998. 92: 3388-93
6. Liu P, Li P, Lei T, Qu L, Huang H, Mu Q. Acute lymphoblastic leukemia following temozolomide treatment in a patient with glioblastoma: A case report and review of the literature. Oncol Lett. 2018. 15: 8663-8
7. Lusis EA, Travers S, Jost SC, Perry A. Glioblastomas with giant cell and sarcomatous features in patients with Turcot syndrome Type 1: A clinicopathological study of 3 cases. Neurosurgery. 2010. 67: 811-7
8. Myong NH, Park BJ. Malignant glioma arising at the site of an excised cerebellar hemangioblastoma after irradiation in a von Hippel-Lindau disease patient. Yonsei Med J. 2009. 50: 576-81
9. Rugała Z. Long-term survival of a patient suffering from glioblastoma multiforme of the brain as well as kidney cancer diagnosed and treated simultaneously. Nowotwory J Oncol. 2018. 68: 268-72
10. Simanjuntak KA, Al Fauzi A, Christi AY, Budiono PS, Susilo RI, Haq IB. Clear-cell renal cell carcinoma and glioblastoma multiforme coexistence: Double primary malignancy, does it have a causal relationship?. Surg Neurol Int. 2022. 13: 361
11. Zhang GT, Liu Q, Zuo FX, Liu HJ, Wang SQ, Yuan Q. Clinical and genomic features in patients with second primary glioblastoma following first primary renal cell carcinoma. BMC Cancer. 2023. 23: 104