- Department of Surgery, College of Medicine, King Saud University, Riyadh, Saudi Arabia
- Department of Neurosurgery, King Saud University, Riyadh, Saudi Arabia.
Correspondence Address:
Aued Iaed Alanazi, College of Medicine, King Saud University, Riyadh, Saudi Arabia.
DOI:10.25259/SNI_555_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Aued Iaed Alanazi1, Tariq Alanezi1, Ziyad Fahad Aljofan1, Alwaleed Alarabi1, Sherif Elwatidy2. Lhermitte–Duclos disease: A systematic review. 29-Sep-2023;14:351
How to cite this URL: Aued Iaed Alanazi1, Tariq Alanezi1, Ziyad Fahad Aljofan1, Alwaleed Alarabi1, Sherif Elwatidy2. Lhermitte–Duclos disease: A systematic review. 29-Sep-2023;14:351. Available from: https://surgicalneurologyint.com/surgicalint-articles/12569/
Abstract
Background: Lhermitte–Duclos disease (LDD) is a rare tumor, with only about 300 reported cases. It often shows comorbidity with Cowden syndrome (CS); however, it can occur by itself. Radiologically, the “tiger-stripe” appearance is considered pathognomonic. Surgical resection remains the mainstay of treatment. This report aims to describe the clinical and radiological characteristics of LDD and its relationship with CS according to age group.
Methods: PubMed electronic databases were searched in August 2022. The search terms included “Lhermitte– Duclos disease” and “dysplastic gangliocytoma,” which yielded 297 and 103 research articles, respectively. The articles were collected and reviewed by three researchers.
Results: Out of 400 identified articles, we analyzed 302 reported cases. The mean age at presentation was 33.6 ± 16 years; 171 patients (56.6%) were female, and 123 (40.7%) were male. The most commonly reported symptom was headache (174 patients, 57.6%), followed by ataxia (109, 36.1%). In addition, 99 cases (32.8%) were associated with CS, and 60 (19.9%) had a confirmed phosphatase and tensin homolog (PTEN) mutation. A tiger-stripe appearance was observed in 208 cases (58.7%); surgical resection was performed in 64.2% of the cases. Mortality and recurrence rates were 4.3% and 8.6%, respectively. No statistically significant difference was found between adult- and pediatric-onset LDD for the association with CS (P = 0.128).
Conclusion: Our findings suggest that adult and pediatric LDD have major commonalities; however, further prospective studies are warranted.
Keywords: Cowden syndrome, Disease onset, Lhermitte–Duclos disease, Phosphatase and tensin homolog
INTRODUCTION
Lhermitte–Duclos disease (LDD) or dysplastic gangliocytoma of the cerebellum is a benign, rare, hamartomatous lesion characterized by a slow and progressive abnormal growth in the cerebellum.[
Given the association between LDD and Cowden syndrome (CS), numerous non-neurological signs and symptoms suggest the existence of such a lesion. LDD was introduced in 1920 by Lhermitte and Duclos,[
Surgical intervention for LDD was described in 1937 by Christensen, who reported a patient with LDD who underwent surgery and was discharged from the hospital without complaints.[
The epidemiology of LDD remains unclear, with over 300 cases being reported worldwide. Most cases occur between the 2nd and 4th decades, with no apparent sex predominance.[
The histopathological characteristics of LDD include the absence of the Purkinje cell layer; increased thickness of the molecular cell layer, which yields axon over-myelinization; hypertrophy of the granular cell layer; replacement of the granular cell layer with dysplastic cortical neurons; and atrophy of cerebellar white matter.[
The management of LDD is usually simple, with surgical resection reserved for symptomatic patients; cerebrospinal fluid (CSF) diversion is performed in patients who develop signs and symptoms of increased intracranial pressure. Systemic chemotherapeutic agents are not prescribed. Although other modalities have been used, including molecularly targeted therapies, evidence remains insufficient.[
CS is a rare, autosomal dominant, multisystem clinical entity characterized by malignant and hamartomatous lesions developing across the body, particularly in the breast, endometrium, and thyroid. CS is caused by a germline mutation in the phosphatase and tensin homolog (PTEN) gene, a tumor suppressor gene found on human chromosome 10q23.[
Although >300 cases of LDD have been reported, research remains insufficient. Specifically, the proportion of cases that recur after surgical resection, the mortality rate of the lesion, and the number of cases associated with CS and PTEN mutations, especially in the pediatric population, remain unclear. This systematic review aimed to describe the clinical characteristics of LDD and its radiological appearance with different conventional MRI sequences. In addition, we aimed to assess the association of adult and pediatric LDD with CS and PTEN mutations, reported surgical and medical management protocols, and the recurrence and mortality rates of LDD.
MATERIALS AND METHODS
Study objectives
The primary study objective was to review the clinical characteristics, radiological appearance, genetic associations, and outcomes in reported cases of LDD. In addition, we aimed to evaluate differences between adult- and pediatric-onset LDD regarding their association with CS.
Search methods
We queried the PubMed database using the search terms “Lhermitte–Duclos disease” and “dysplastic gangliocytoma,” yielding 297 and 103 articles, respectively. The study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.
Inclusion and exclusion criteria
We did not include the first report of LDD by Lhermitte and Duclos[
Selection of studies
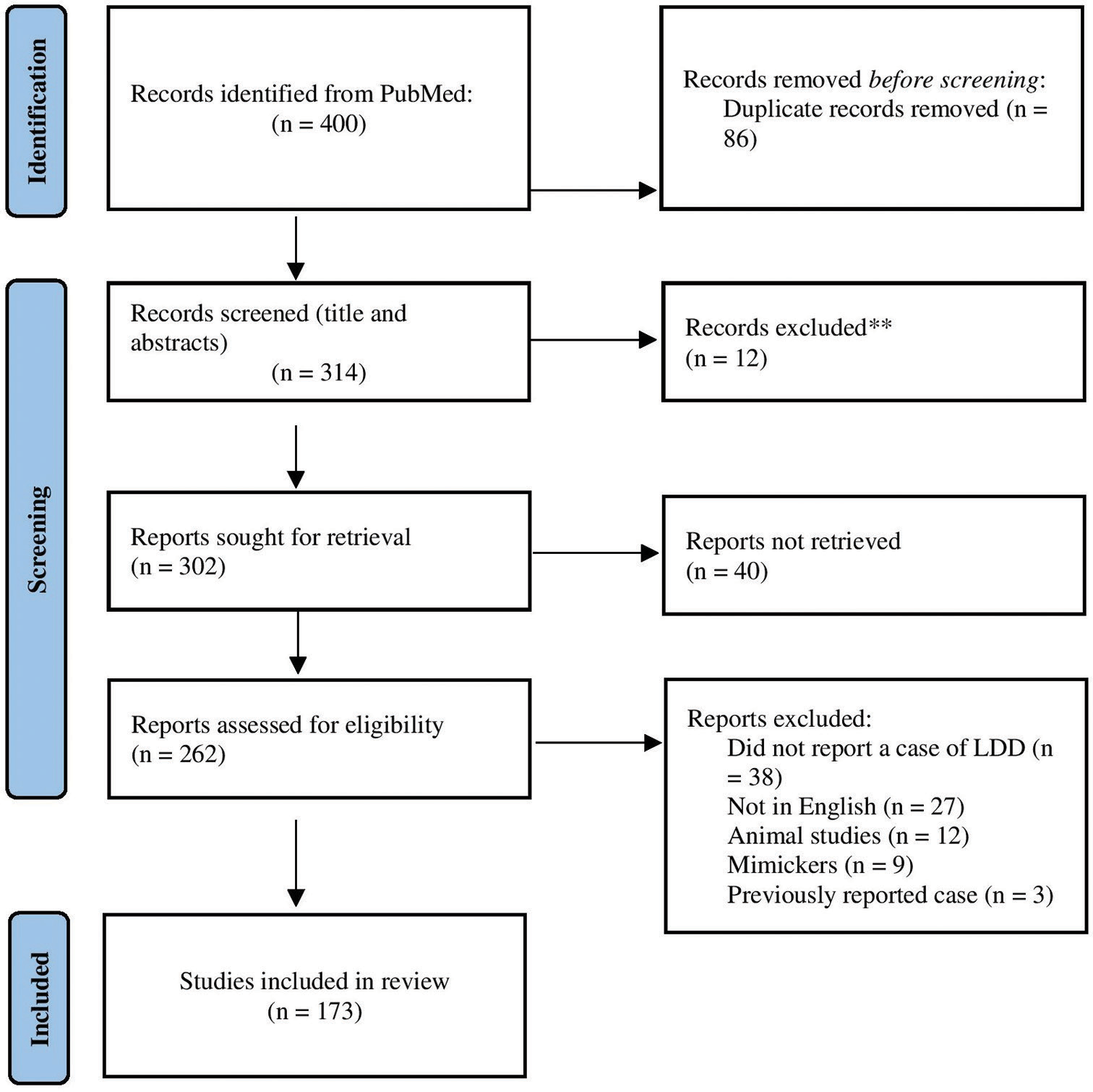
The eligibility of identified articles was independently evaluated by three authors (AA, TA, and ZA) through an initial screening of the title and abstract, followed by a full-text review. We included all studies that reported relevant information regarding the predetermined set of variables. The study excluded articles lacking the given variables, non-human cases, non-English articles, LDD-mimicking cases, and reviews that did not report a new case. In addition, we excluded all cases in which data could not be retrieved. Disagreements were resolved by agreement of at least two of the three reviewers [
Data collection
We extracted the following data from the original articles: demographic data, clinical presentation, CS status, presence of PTEN mutations, radiological presentation, management protocol, recurrence, and mortality.
RESULTS
We extracted 302 reported cases from the 400 identified articles. Demographic, clinical, and radiological data were collected from original articles. Descriptive analyses were performed using IBM SPSS Statistics for Windows version 25. Fisher’s exact test was performed to assess differences between adult- and child-onset LDD related to CS, recurrence, and mortality rates.
Demographics
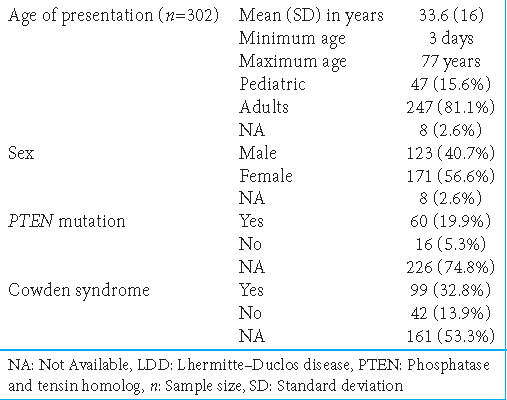
At presentation, the mean age (± standard deviation) was 33.6 ± 16 years, ranging between 3 days and 77 years. We divided patients into two groups based on their age; specifically, patients aged <18 years and >18 years were included in the pediatric (47 patients, 15.6%) and adult (247 patients, 81.8%) groups, respectively, with only eight patients (2.6%) having data missing for age. For sex distribution, female patients (171, 56.6%) outnumbered male patients (123, 40.7%) [
CS and PTEN mutation
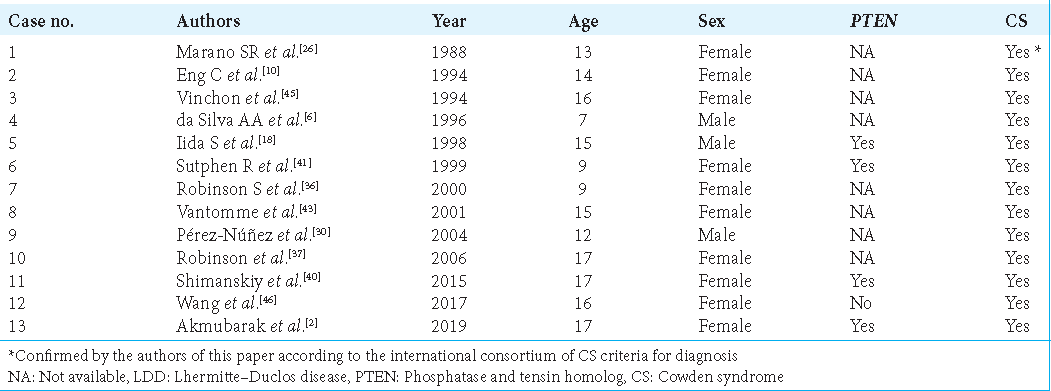
LDD was associated with CS in 99 patients (32.8%) and not associated in 42 (13.9%); 161 patients (53.3%) lacked data. Moreover, LDD was associated with PTEN mutations in 60 cases (19.9%) and not associated and 42 (13.9%); 161 cases (53.3%) lacked relevant information [
Clinical presentation
Regarding the clinical presentation of LDD, the most common symptom was headache (“occipital” or “global;” n = 174, 57.6%), followed by ataxia (n = 109, 36.1%); cerebellar signs, including dysmetria and dysdiadochokinesia (n = 68, 22.5%); papilledema (n = 61, 20.2%); visual disturbances (n = 51, 16.9%); dizziness (n = 40, 13.2%); nausea (n = 36, 11.9%); vomiting (n = 35, 11.6%); diplopia and strabismus, usually involving eye abduction secondary to abducens cranial nerve palsy and elevated intracranial pressure (n = 29, 9.6%); nystagmus (n = 24, 7.9%); vertigo (n = 22, 7.3%); and altered mental status (n = 16, 5.3%). Several cases did not mention the patient’s clinical presentation; 13 cases (4.3%) reported them incidentally [
Radiological presentation
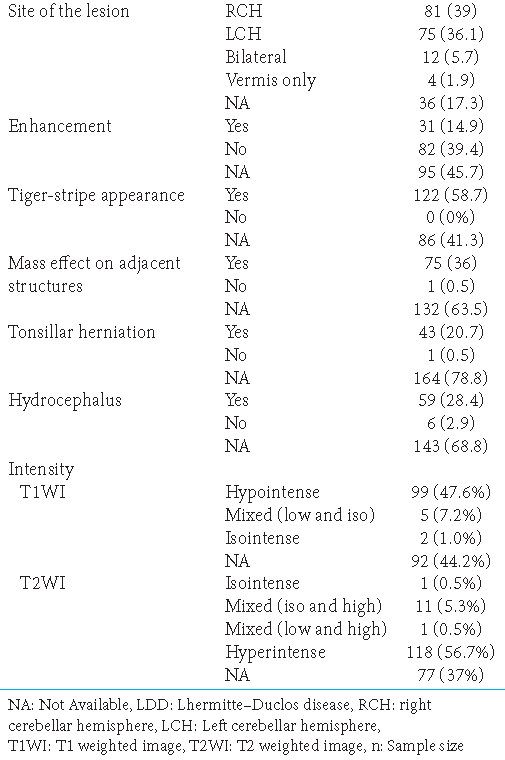
Among the 302 cases, only 208 reported radiological findings; the reported lesions were usually unilateral. The right cerebellar hemisphere (n = 81, 39%) was more affected than the left (n = 75, 36.1%). Only 12 patients (5.7%) presented with bilateral lesions; 4 (1.9%) had midline lesions involving only the vermis. The lesion site was not described in 36 cases (17.3%). Enhancement patterns (usually mild) were present in 31 patients (14.9%) and absent in 82 (39.4%); 95 (45.7%) cases lacked relevant information.
Regarding the characteristic findings of LDD, a classic tiger-stripe appearance was described in only 122 cases (58.7%). The mass effect on adjacent structures was present in 75 cases (36%) and absent in 1 case (0.5%); the other cases lacked relevant data (63.5%). Tonsillar herniation was reported in 43 cases (20.7%); similar results were found for hydrocephalus, with 59 patients (28.4%) presenting radiological signs of increased intracranial pressure. Six reports denied any apparent hydrocephalus; 143 reports had no relevant data.
Signal intensity of LDD in conventional MRI
Regarding intensity, the lesion had a low signal on T1WI in 99 cases (47.6%), a mixture of low and isointensity in 5 cases (7.2%), and a solely isointense pattern in 2 cases (1%). No relevant data were available regarding T1WI in 92 cases (44.2%). On T2WI, isointensity was reported in only 1 patient (0.5%). In most cases (n = 118, 56.7%), the lesion appeared hyperintense. Mixed intensities were reported in two different presentations: mixed isointensity and hyperintensity (n = 11, 5.3%) and mixed hypo- and hyper-intensity (n = 1, 0.5%) [
Management of LDD
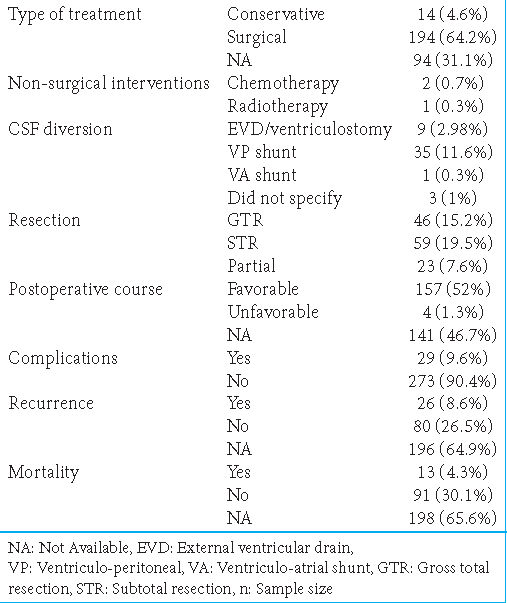
Conservative management was performed in 14 patients (4.6%), with many refusing treatment. Conservative management included using analgesics, antiemetics, and radiological monitoring (MRI). The type of management was not described for 94 patients (31.1%). Surgical intervention was performed in 194 patients (64.2%). Non-surgical interventions were also reported: 2 patients (0.7%) received chemotherapy and 1 (0.3%) received radiotherapy. CSF diversion was established in 48 patients (15.9%), with the most common method being ventriculoperitoneal shunting (n = 35, 11.6%), followed by external ventricular draining (n = 9, 3%) and ventriculoatrial shunting (n = 1, 0.3%). The CSF diversion method was not specified in 3 cases (1%).
The extent of resection was defined in three different categories. Specifically, gross-total resection (GTR), subtotal resection (STR), and partial resection were indicated by resection of ~100%, 50–100%, and <50% of the lesion, respectively. GTR, STR, and partial resection were reported in 46 (15.2%), 59 (19.5%), and 23 (7.6%) patients, respectively [
Postoperative course
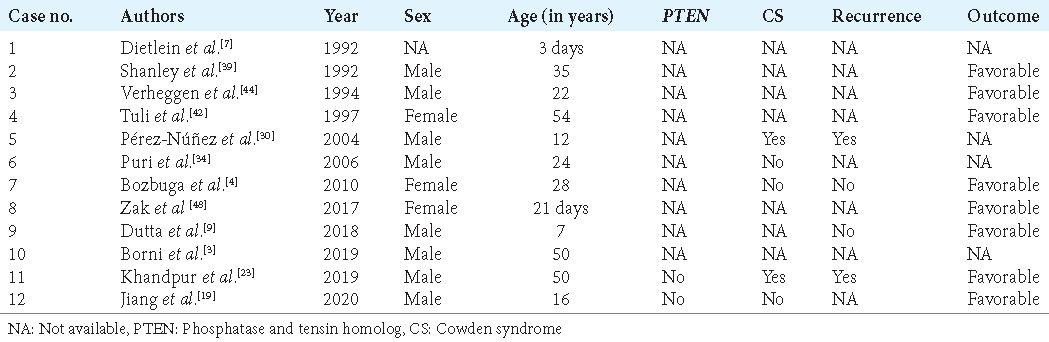
Favorable and unfavorable outcomes were reported in 157 (52%) and 4 (1.3%) cases, respectively; the remaining cases had no data regarding the postoperative course. The complication rate was 9.6% (29 patients). Recurrence was observed in 26 patients (8.6%), among whom 11 were associated with CS. Recurrence was not observed in 80 patients (26.5%); 196 cases (64.9%) lacked information regarding recurrence. Death was reported in only 13 patients (4.3%); the remaining cases denied death (n = 91, 30.1%) or had no data regarding death (n = 198, 65.6%) [
The most common complications were persistent cerebellar symptoms (n = 8, 2.65%), followed by seizures (n = 5, 1.66%) and cerebellar mutism and respiratory difficulty (n = 3, 1%). Hydrocephalus, shunt infection, and ventriculitis were observed in 2 patients (0.7%) each. Other rare complications included encephalomalacia, diabetes insipidus, global cerebral ischemia, vision deterioration, and hypotension [
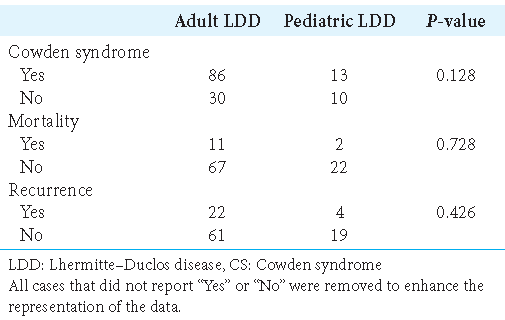
LDD and age-related association with CS
No significant differences were found between adult- and pediatric-onset LDD for association with CS, mortality rate, and lesion recurrence, with P = 0.128, 0.728, and 0.426, respectively [
DISCUSSION
Since the first case of LDD was reported in 1920 by Lhermitte and Duclos,[
Age, sex, and demographics
The mean age of presentation was consistent with LDD occurring mainly in the 2nd and 4th decades of life.[
CS and genetic association
LDD was associated with CS and PTEN mutations in one-third and one-fifth of cases, respectively. However, many of these cases were diagnosed before the international consortium of CS criteria for diagnosis was developed.[
Pérez-Núñez et al.[
CS and the onset of LDD
The association of CS with LDD is further complicated by the distinction between adult- and childhood-onset LDD. Zhou et al.[
In addition, no significant difference between adult and pediatric patients was found for the prevalence of CS, recurrence, or mortality. However, given the heterogeneity of the reported data, the retrospective nature of this review, and the short follow-up duration, we could not determine the exact differences between these age groups. Moreover, definitions for the pediatric population have been inconsistent. In our review, we applied the relatively common definition of an age <18 years for the pediatric population. Zhou et al. described 18 patients, among whom 15 had confirmed germline mutations in the PTEN gene, and all were adults. This result demonstrates the importance of differentiating between adult- and pediatric-onset LDD. On immunohistochemistry, 75% of cases showed a loss of PTEN protein expression.[
Bilateral involvement and the genetic origins of LDD
This review identified 46 patients with CS and PTEN mutations, representing 46.5% of those with both LDD and CS. This result is consistent with the previous reports and supports the overall premise of focusing less on the role of PTEN in the pathogenesis of isolated LDD.[
Radiological appearance
The appearance of LDD is usually described as a non-enhancing, well-circumscribed lesion, unilateral in the cerebellum, with low signal on T1WI, high signal on T2WI, and fluid-attenuated inversion recovery, and presenting the tiger-stripe appearance.[
Tiger-stripe appearance and enhancement
This review is the first large-scale report on the prevalence of enhancement, tiger-stripe appearance, and mass effect of LDD on MRI. The finding that lesion enhancements and linear striations were observed in 14.9% and 58.7% of cases is consistent with a previous small-scale review of 21 patients that found that 61.9% of the patients manifested the classical layered pattern.[
Enhancement is not rare in LDD. Different enhancement patterns were observed in 14.9% of cases; however, most had mild enhancement. In the aforementioned review, only 5 patients (23.8%) showed heterogeneous enhancement,[
Magnetic resonance spectroscopy (MRS) and LDD malignancy
MRS can assess the chemical content of a given lesion, enabling differentiation of malignancy degrees through the measurement of chemical metabolites such as choline, N-acetylaspartate (NAA), and lactate.[
Zhang et al.[
Management, postoperative course, and recurrence
Most patients in our review (64.2%) underwent surgical management, with 19.5% undergoing STR. The extent of resection seems to have a strong correlation with LDD recurrence.[
Of the 26 patients that experienced recurrence, 11 had a CS mutation and one had a PTEN mutation; 15 underwent subtotal or partial resection, four underwent GTR, and seven lacked information regarding the extent of resection. After treatment, four patients died, and seven experienced posttreatment complications.
Nonsurgical interventions and conservative management
Chemotherapy and radiotherapy were reported in two and one case, respectively. One patient had received ten cycles of metronomic temozolomide over 12 months; the patient developed nausea and vomiting. The second patient received rapamycin, a first-generation mechanistic target of rapamycin (mTOR) kinase inhibitor. The mTOR protein controls cell growth, survival, and proliferation.[
Limitations of this study
First, given the rarity of LDD, most of the literature consists of subjective reports of isolated cases. Second, we could not establish causal relationships due to the heterogeneity of the reported data, the review’s retrospective nature, and the rarity of the disease. Further, research should investigate the differences between pediatric- and adult-onset LDD regarding CS association and follow-up. Moreover, the lack of consensus on the diagnosis of CS and differences in the definition of pediatric populations further restrict our understanding of LDD.
CONCLUSION
LDD is a rare tumor with many uncertainties and mimickers but generally has a good prognosis. One-third of the cases are associated with CS, and a highly specific radiological appearance can sometimes be observed. This systematic review found no apparent differences between adult- and pediatric-onset LDD for their association with CS. However, further prospective evaluations of this association should be conducted to enhance understanding of this disease.
Declaration of patient consent
Patients’ consent not required as patients’ identities were not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The author(s) confirms that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Abel TW, Baker SJ, Fraser MM, Tihan T, Nelson JS, Yachnis AT. Lhermitte-Duclos disease: A report of 31 cases with immunohistochemical analysis of the PTEN/AKT/mTOR pathway. J Neuropathol Exp Neurol. 2005. 64: 341-9
2. Almubarak AO, Haq AU, Alzahrani I, Shail EA. LhermitteDuclos disease with cervical arteriovenous fistula. J Neurol Surg A Cent Eur Neurosurg. 2019. 80: 134-7
3. Borni M, Kammoun B, Kolsi F, Abdelmouleh S, Boudawara MZ. The Lhermitte-Duclos disease: A rare bilateral cerebellar location of a rare pathology. Pan Afr Med J. 2019. 33: 118
4. Bozbuga M, Gulec I, Suslu HT, Bayindir C. Bilateral LhermitteDuclos disease. Neurol India. 2010. 58: 309-11
5. Christensen E. About ganglion cell tumors in the brain. Virchows Arch Pathol Anat Physiol Klin Med. 1937. 300: 567-81
6. da Silva AA, Banerjee T, Coimbra RL. Lhermitte-Duclos disease (cerebellar gangliocytoma). South Med J. 1996. 89: 1208-12
7. Dietlein M, Schröder R, Widemann B, Benz-Bohm G. Dysplastic gangliocytoma of cerebellum in a newborn. Diagnosis by ultrasonography and MRI. Pediatr Radiol. 1992. 22: 131-3
8. Douglas-Akinwande AC, Payner TD, Hattab EM. Medulloblastoma mimicking Lhermitte-Duclos disease on MRI and CT. Clin Neurol Neurosurg. 2009. 111: 536-9
9. Dutta G, Singh D, Saran RK, Singh H, Srivastava AK, Jagetia A. Childhood Lhermitte-Duclos disease progressing to medulloblastoma in bilateral cerebellar hemispheres: Report of unusual case. World Neurosurg. 2018. 117: 344-9
10. Eng C, Murday V, Seal S, Mohammed S, Hodgson SV, Chaudary MA. Cowden syndrome and Lhermitte-Duclos disease in a family: A single genetic syndrome with pleiotropy?. J Med Genet. 1994. 31: 458-61
11. Eng C. Will the real Cowden syndrome please stand up: Revised diagnostic criteria. J Med Genet. 2000. 37: 828-30
12. Ezgu MC, Ozer MI, Dogan A, Deveci G, Kural C, Izci Y. Lhermitte-Duclos disease in a six-year old child: A rare presentation. Pediatr Neurosurg. 2018. 53: 416-20
13. Farooq A, Walker LJ, Bowling J, Audisio RA. Cowden syndrome. Cancer Treat Rev. 2010. 36: 577-83
14. Georgescu MM. PTEN tumor suppressor network in PI3K-Akt pathway control. Genes Cancer. 2010. 1: 1170-7
15. Gharzeddine K, Hatzoglou V, Holodny AI, Young RJ. MR perfusion and MR spectroscopy of brain neoplasms. Radiol Clin North Am. 2019. 57: 1177-88
16. Han C, Zhang Y, Ran C, Luo Y, Li W. Diverse imaging findings of Lhermitte-Duclos disease. Clin Radiol. 2023. 78: 33-9
17. Hariri OR, Khachekian A, Muilli D, Amin J, Minassian T, Berman B. Acute-onset cerebellar symptoms in Lhermitte-Duclos disease: Case report. Cerebellum. 2013. 12: 127-30
18. Iida S, Tanaka Y, Fujii H, Hayashi S, Kimura M, Nagareda T. A heterozygous frameshift mutation of the PTEN/ MMAC1 gene in a patient with Lhermitte-Duclos disease-only the mutated allele was expressed in the cerebellar tumor. Int J Mol Med. 1998. 1: 925-9
19. Jiang C, Lu WX, Yan GZ, Bai RB, Wang ZN, Chen Y. Bilateral dysplastic gangliocytoma with concurrent polyostotic fibrous dysplasia: A case report and literature review. World Neurosurg. 2020. 141: 421-4
20. Jiang T, Wang J, Du J, Luo S, Liu R, Xie J. LhermitteDuclos disease (dysplastic gangliocytoma of the cerebellum) and Cowden syndrome: Clinical experience from a single institution with long-term follow-up. World Neurosurg. 2017. 104: 398-406
21. Johnston JM, Limbrick DD, Ray WZ, Brown S, Shimony J, Park TS. Isolated cerebellar Rosai-Dorfman granuloma mimicking Lhermitte-Duclos disease. J Neurosurg Pediatr. 2009. 4: 118-20
22. Joo GJ, Doumanian J. Radiographic findings of dysplastic cerebellar gangliocytoma (Lhermitte-Duclos disease) in a woman with Cowden syndrome: A case study and literature review. J Radiol Case Rep. 2020. 14: 1-6
23. Khandpur U, Huntoon K, Smith-Cohn M, Shaw A, Elder JB. Bilateral recurrent dysplastic cerebellar gangliocytoma (Lhermitte-Duclos disease) in cowden syndrome: A case report and literature review. World Neurosurg. 2019. 127: 319-25
24. Kumar R, Vaid VK, Kalra SK. Lhermitte-Duclos disease. Childs Nerv Syst. 2007. 23: 729-32
25. Lhermitte J, Duclos P. Sur un ganglioneurome diffus du cortex du cervelet. Bull Assoc Franc Etude Cancer. 1920. 9: 99-107
26. Marano SR, Johnson PC, Spetzler RF. Recurrent LhermitteDuclos disease in a child. J Neurosurg. 1988. 69: 599-603
27. Nair P, Pal L, Jaiswal AK, Behari S. Lhermitte-Duclos disease associated with dysembryoplastic neuroepithelial tumor differentiation with characteristic magnetic resonance appearance of “tiger striping”. World Neurosurg. 2011. 75: 699-703
28. Nowak DA, Trost HA, Porr A, Stölzle A, Lumenta CB. Lhermitte-Duclos disease (Dysplastic gangliocytoma of the cerebellum). Clin Neurol Neurosurg. 2001. 103: 105-10
29. Nowak DA, Trost HA. Lhermitte-Duclos disease (dysplastic cerebellar gangliocytoma): A malformation, hamartoma or neoplasm?. Acta Neurol Scand. 2002. 105: 137-45
30. Pérez-Núñez A, Lagares A, Benítez J, Urioste M, Lobato RD, Ricoy JR. Lhermitte-Duclos disease and Cowden disease: Clinical and genetic study in five patients with LhermitteDuclos disease and literature review. Acta Neurochir (Wien). 2004. 146: 679-90
31. Pilarski R, Burt R, Kohlman W, Pho L, Shannon KM, Swisher E. Cowden syndrome and the PTEN hamartoma tumor syndrome: Systematic review and revised diagnostic criteria. J Natl Cancer Inst. 2013. 105: 1607-16
32. Pilarski R, Stephens JA, Noss R, Fisher JL, Prior TW. Predicting PTEN mutations: An evaluation of Cowden syndrome and Bannayan-Riley-Ruvalcaba syndrome clinical features. J Med Genet. 2011. 48: 505-12
33. Pilarski R. PTEN hamartoma tumor syndrome: A clinical overview. Cancers (Basel). 2019. 11: 844
34. Puri AS, Garg A, Mishra NK, Gaikwad SB, Mehta VS, Cirillo S. Bilateral cerebellar dysplastic gangliocytomas (Lhermitte Duclos disease) with cerebellar ectopia and presyrinx cord changes. Neuroradiol J. 2006. 19: 717-21
35. Reeder RF, Saunders RL, Roberts DW, Fratkin JD, Cromwell LD. Magnetic resonance imaging in the diagnosis and treatment of Lhermitte-Duclos disease (dysplastic gangliocytoma of the cerebellum). Neurosurgery. 1988. 23: 240-5
36. Robinson S, Cohen AR. Cowden disease and LhermitteDuclos disease: Characterization of a new phakomatosis. Neurosurgery. 2000. 46: 371-83
37. Robinson S, Cohen AR. Cowden disease and Lhermitte-Duclos disease: An update. Case report and review of the literature. Neurosurg Focus. 2006. 20: E6
38. Sasongko TH, Ismail NF, Zabidi-Hussin Z. Rapamycin and rapalogs for tuberous sclerosis complex. Cochrane Database Syst Rev. 2016. 7: CD011272
39. Shanley DJ, Vassallo CJ. Atypical presentation of LhermitteDuclos disease: Preoperative diagnosis with MRI. Neuroradiology. 1992. 34: 103-4
40. Shimanskiy VN, Karnaukhov VV, Shishkina LV, Vinogradov EV. The successful treatment of a patient with Lhermitte-Duclos disease (A case report and literature rewiew). Zh Vopr Neirokhir Im N N Burdenko. 2015. 79: 78-83
41. Sutphen R, Diamond TM, Minton SE, Peacocke M, Tsou HC, Root AW. Severe Lhermitte-Duclos disease with unique germline mutation of PTEN. Am J Med Genet. 1999. 82: 290-3
42. Tuli S, Provias JP, Bernstein M. Lhermitte-Duclos disease: Literature review and novel treatment strategy. Can J Neurol Sci. 1997. 24: 155-60
43. Vantomme N, Van Calenbergh F, Goffin J, Sciot R, Demaerel P, Plets C. Lhermitte-Duclos disease is a clinical manifestation of Cowden’s syndrome. Surg Neurol. 2001. 56: 201-4
44. Verheggen R, Bruhn H, Schröder BU, Frahm J, Markakis E. Lhermitte-Duclos disease: A critical appraisal of different radiologic methods. Eur J Radiol. 1994. 19: 21-4
45. Vinchon M, Blond S, Lejeune JP, Krivosik I, Fossati P, Assaker R. Association of Lhermitte-Duclos and Cowden disease: Report of a new case and review of the literature. J Neurol Neurosurg Psychiatry. 1994. 57: 699-704
46. Wang Q, Zhang S, Cheng J, Liu W, Hui X. Lhermitte-Duclos disease: Clinical study with long-term follow-up in a single institution. Clin Neurol Neurosurg. 2017. 162: 53-8
47. Xu L, Gao PY, Lin Y, Tian TD, Lei J, Ma L. Magnetic resonance imaging findings in Lhermitte-Duclos disease: Reports of three cases. Chin Med J (Engl). 2005. 118: 1933-6
48. Zak M, Ledbetter M, Maertens P. Infantile Lhermitte-Duclos disease treated successfully with rapamycin. J Child Neurol. 2017. 32: 322-6
49. Zhang HW, Zhang YQ, Liu XL, Mo YQ, Lei Y, Lin F. MR imaging features of Lhermitte-Duclos disease. Medicine (Baltimore). 2022. 101: e28667
50. Zhou XP, Marsh DJ, Morrison CD, Chaudhury AR, Maxwell M, Reifenberger G. Germline inactivation of PTEN and dysregulation of the phosphoinositol-3-kinase/Akt pathway cause human Lhermitte-Duclos disease in adults. Am J Hum Genet. 2003. 73: 1191-8