- Department of Neurosurgery, Kanazawa University Hospital, Kanazawa, Ishikawa, Japan.
Correspondence Address:
Masahiro Oishi, Department of Neurosurgery, Kanazawa University Hospital, Kanazawa, Ishikawa, Japan.
DOI:10.25259/SNI_937_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Munehiro Demura, Masahiro Oishi, Naoyuki Uchiyama, Masanao Mohri, Katsuyoshi Miyashita, Mitsutoshi Nakada. Limb-shaking syndrome derived from the contralateral hemisphere following unilateral revascularisation for moyamoya disease. 23-Nov-2021;12:579
How to cite this URL: Munehiro Demura, Masahiro Oishi, Naoyuki Uchiyama, Masanao Mohri, Katsuyoshi Miyashita, Mitsutoshi Nakada. Limb-shaking syndrome derived from the contralateral hemisphere following unilateral revascularisation for moyamoya disease. 23-Nov-2021;12:579. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=11236
Abstract
Background: Moyamoya disease is a rare chronic steno-occlusive cerebrovascular disease. It may have variable clinical symptoms associated with cerebral stroke, including motor paralysis, sensory disturbances, seizures, or headaches. However, patients with moyamoya disease rarely present with involuntary movement disorders, including limb-shaking syndrome, with no previous reports of limb-shaking syndrome occurring after revascularization procedures for this disease. Although watershed shifts can elicit transient neurological deterioration after revascularisation, symptoms originating from the contralateral hemisphere following the revascularization procedure are rare. Here, we report the case of moyamoya disease wherein the patient developed limb-shaking syndrome derived from the contralateral hemisphere after unilateral revascularisation.
Case Description: A 16-year-old girl presented with transient left upper and lower limb numbness and headache. Based on digital subtraction angiography, she was diagnosed with symptomatic moyamoya disease. Single-photon emission computed tomography (SPECT) showed decreased cerebral blood flow (CBF) on the right side, and she underwent direct and indirect bypasses on this side. Involuntary movements appeared in her right upper limb immediately postoperatively. SPECT showed decreased CBF to the bilateral frontal lobes. Subsequently, the patient was diagnosed with limb-shaking syndrome. After performing left-hemispheric revascularisation, the patient’s symptoms resolved, and SPECT imaging confirmed improvements in CBF to the bilateral frontal lobes.
Conclusion: Revascularization for moyamoya disease can lead to watershed shifts, which can induce limb-shaking syndrome derived from abnormalities in the contralateral hemisphere of the revascularized side. For patients with new-onset limb-shaking syndrome after moyamoya revascularisation procedures, additional revascularization may be warranted for treatment of low perfusion areas.
Keywords: Limb-shaking syndrome, Moyamoya disease, Postoperative involuntary movement, Watershed shift
INTRODUCTION
Moyamoya disease is characterized by progressive steno-occlusive changes at the terminal portions of the internal carotid artery (ICA) and the development of moyamoya vessels. Patients with moyamoya disease usually present with clinical symptoms associated with ischaemic attacks and intracranial hemorrhages, including motor paralysis, sensory disturbances, seizures, or headaches.[
Although the most effective treatment for moyamoya disease is revascularization surgery, postoperative complications such as ischemic/hemorrhagic stroke or cerebral hyperperfusion syndrome have been reported.[
Herein, we present the case of limb-shaking syndrome derived from the contralateral hemisphere after a unilateral revascularisation procedure for moyamoya disease.
CASE REPORT
A 16-year-old girl presented with transient left upper and lower limb numbness and headache while participating in chorus and sports. She was referred to our department for surgical treatment. She had a past medical history of immunoglobulin A nephropathy and was being treated with steroids. Magnetic resonance (MR) images showed no ischaemic changes in her brain, but MR angiography showed intracranial vascular stenosis [
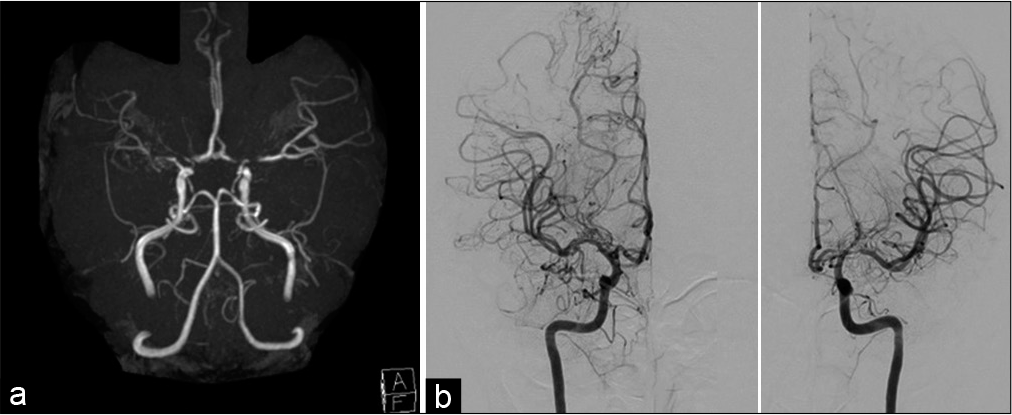
Figure 1:
Patient angiography findings at initial presentation. (a) Intracranial vascular stenosis was identified on magnetic resonance angiography. (b) Digital subtraction angiography suggested bilateral stenoses from the terminal portion of the internal carotid artery to the proximal parts of the middle and anterior cerebral arteries, with extended lenticulostriate arteries.
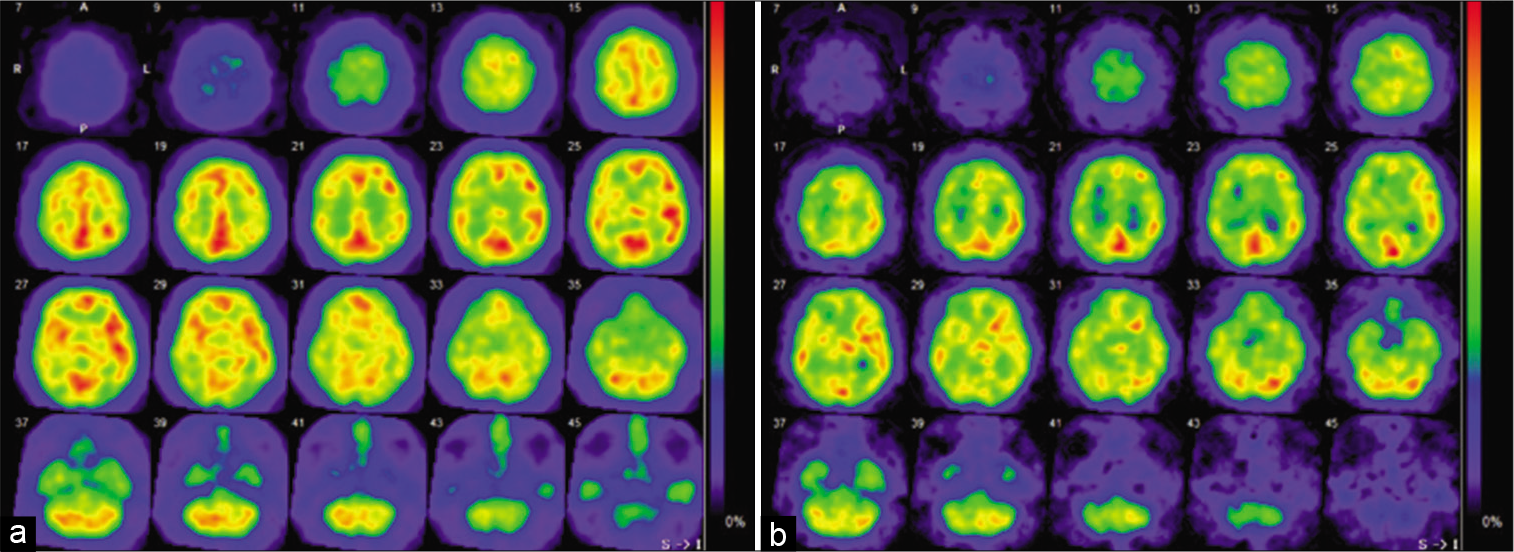
Figure 2:
Preoperative single-photon emission computed tomography (SPECT) imaging. (a) N-isopropyl-p-[123I] iodoamphetamine SPECT showed decreased cerebral blood flow (CBF) in the right hemisphere at rest, preoperatively. (b) Paradoxical reduction of CBF after acetazolamide administration was identified in the right hemisphere before surgery.
During surgery, both direct and indirect bypasses were successfully performed; however, involuntary movements appeared immediately after the operation, with her right upper limb episodically shaking at a frequency of 3 to 5 Hz [
Video 1
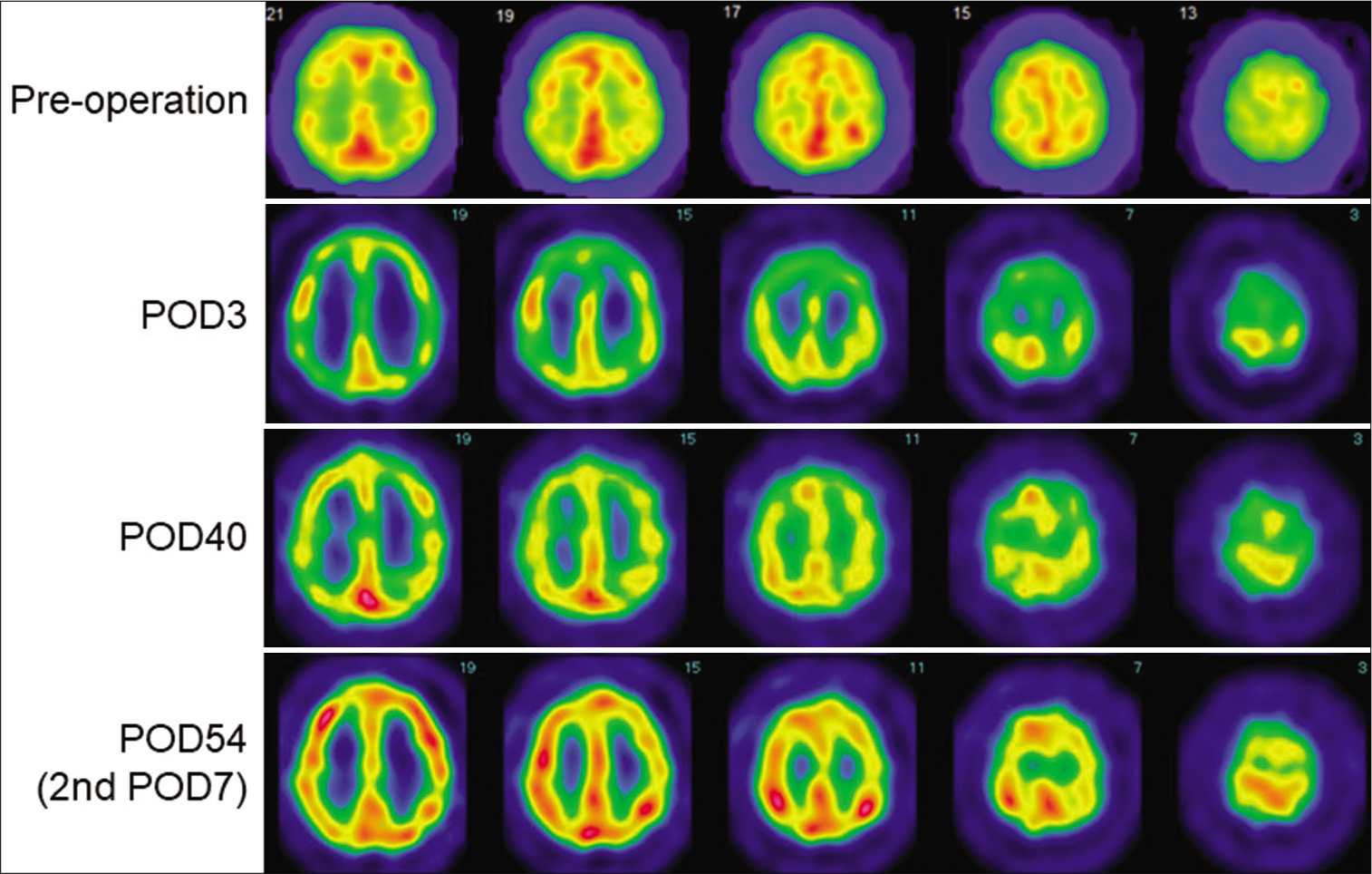
Figure 4:
Postoperative single-photon emission computed tomography (SPECT). Technetium-99m ethyl cysteinate dimer (99mTc-ECD) SPECT on postoperative day (POD) 3 did not show hyperperfusion in the right hemisphere but did show decreased cerebral blood flow (CBF) in the bilateral frontal lobe areas. Forty days after surgery, hypoperfusion of bilateral frontal lobes on SPECT was slightly improved but remained to a lesser extent. Seven days after the second revascularisation procedure, 123I-IMP SPECT showed improvement of whole-brain CBF, including in both frontal lobes.
The frequency of her involuntary movements gradually decreased, and SPECT imaging showed slight improvement of the hypoperfusion in her bilateral frontal lobes; however, this symptom and her abnormal imaging findings persisted, though to a lesser degree. Therefore, a left-sided revascularisation procedure was performed on postoperative day 47. The following day, her symptoms disappeared and did not recur since then. Furthermore, blood flow in the bilateral frontal lobes was improved according to SPECT imaging performed 7 days after the second surgery [
DISCUSSION
Involuntary movements are rarely associated with moyamoya disease. Pandey et al.[
Limb-shaking syndrome is an involuntary movement disorder first reported by Fisher in 1962.[
Rather than an epileptic disorder, limb-shaking syndrome is considered an ischaemic disorder caused by ICA occlusion or stenosis.[
The watershed-shift phenomenon has recently been shown to cause transient neurological deterioration after revascularization procedures for moyamoya disease. This phenomenon was defined by Hayashi et al.[
Our case suggests that watershed shifts occurring after unilateral revascularization procedures in patients with moyamoya disease may lead to perfusion changes in the contralateral hemisphere and may result in limb-shaking syndrome. In these cases, an additional bypass surgery may be needed as soon as possible.
CONCLUSION
Revascularization for moyamoya disease may cause limb-shaking syndrome by inducing CBF reductions in the contralateral frontal lobe due to watershed shifts. For patients with moyamoya disease who develop new involuntary movements after surgery, additional revascularization procedures may be warranted in areas where CBF reduction is confirmed using SPECT.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Videos available on:
References
1. Fisher CM. Concerning recurrent transient cerebral ischemic attacks. Can Med Assoc J. 1962. 86: 1091-9
2. Fujimura M, Mugikura S, Kaneta T, Shimizu H, Tominaga T. Incidence and risk factors for symptomatic cerebral hyperperfusion after superficial temporal artery-middle cerebral artery anastomosis in patients with moyamoya disease. Surg Neurol. 2009. 71: 442-7
3. Hayashi T, Shirane R, Fujimura M, Tominaga T. Postoperative neurological deterioration in pediatric moyamoya disease: Watershed shift and hyperperfusion. J Neurosurg. 2010. 6: 73-81
4. Ho SC, Lin HJ, Tsui YK, Yeh PS. Limb-shaking TIA related to moyamoya disease: Diagnosis with magnetic resonance imaging and magnetic resonance angiography. Acta Neurol Taiwan. 2010. 19: 270-4
5. Ichiro D, Yoshihiko N, Mikiko N, Toshimasa Y, Naotoshi T, Nobuo A. A case of cerebral infarction caused by occlusion of the left internal carotid artery following limb shaking of the right arm and leg that persisted for three weeks. Jpn J Stroke. 2011. 33: 246-50
6. Im SH, Oh CW, Kwon OK, Cho BK, Chung YS, Han DH. Involuntary movement induced by cerebral ischemia: Pathogenesis and surgical outcome. J Neurosurg. 2004. 100: 877-82
7. Kazumata K, Ito M, Tokairin K, Ito Y, Houkin K, Nakayama N. The frequency of postoperative stroke in moyamoya disease following combined revascularization: A single university series and systematic review. J Neurosurg. 2014. 121: 432-40
8. Kim HY, Chung CS, Lee J, Han DH, Lee KH. Hyperventilation-induced limb shaking TIA in Moyamoya disease. Neurology. 2003. 60: 137-9
9. Kimitoshi S, Shun T, Takanori M, Kobayashi N, Yamashita T, Imataka K. A case of moyamoya disease with postoperative cerebral hyperperfusion syndrome followed by cerebral infarction due to watershed shift. No Shinkei Geka. 2018. 46: 123-9
10. Pandey P, Bell-Stephens T, Steinberg GK. Patients with moyamoya disease presenting with movement disorder. J Neurosurg Pediatrics. 2010. 6: 559-66
11. Persoon S, Kappelle LJ, Klijn CJ. Limb-shaking TIA’s. Tijdschr Neurol Neurochir. 2013. 114: 5-10
12. Schulz UG, Rothwell PM. Transient ischaemic attacks mimicking focal motor seizures. Postgrad Med J. 2002. 78: 246-7
13. Suzuki J, Takaku A. Cerebrovascular “moyamoya” disease. Disease showing abnormal net-like vessels in base of brain. Arch Neurol. 1969. 20: 288-99
14. Taniguchi D, Oji Y, Ueno Y, Hirayama S, Fukui M, Miyamoto N. Limb-shaking transient ischemic attack induced by middle cerebral artery dissection after lung surgery. J Stroke Cerebrovasc Dis. 2017. 26: e197-8
15. Tatemichi TK, Young WL, Prohovnik I, Gitelman DR, Correll JW, Mohr JP. Perfusion insufficiency in limb-shaking transient ischemic attacks. Stroke. 1990. 21: 341-7
16. Tokunaga K, Date I. Moyamoya disease. Brain Nerve. 2008. 60: 37-42
17. Tu XK, Fujimura M, Rashad S, Mugikura S, Sakata H, Niizuma K. Uneven cerebral hemodynamic change as a cause of neurological deterioration in the acute stage after direct revascularization for moyamoya disease: Cerebral hyperperfusion and remote ischemia caused by the ‘watershed shift’. Neurosurg Rev. 2017. 40: 507-12