- Department of Neurosurgery, Epilepsy Clinic, Neurological Center, ABC Medical Center, Mexico City, Mexico.
- Departament of Pediatric Neurosurgery, Epilepsy Clinic, Neurological Center, ABC Medical Center, Mexico City, Mexico.
- Department of Neuroanesthesiology, National Institute of Neurology and Neurosurgery, Mexico City, Mexico.
- Departament of Clinical Neurophysiology, Epilepsy Clinic, Neurological Center, ABC Medical Center, Mexico City, Mexico.
- Pediatric Neurology, Epilepsy Clinic, Neurological Center, ABC Medical Center, Mexico City, Mexico.
Correspondence Address:
Enrique de Font-Réaulx, Department of Neurosurgery, Epilepsy Clinic, Neurological Center, ABC Medical Center, Mexico City, Mexico.
DOI:10.25259/SNI_1169_2021
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Enrique de Font-Réaulx1, Javier Terrazo-Lluch2, Luis Guillermo Díaz-López3, Miguel Ángel Collado-Corona4, Paul Shkurovich-Bialik4, Adalberto González-Astiazarán5. Localization of irritative zones in epilepsy with thermochromic silicone. 12-Jan-2022;13:14
How to cite this URL: Enrique de Font-Réaulx1, Javier Terrazo-Lluch2, Luis Guillermo Díaz-López3, Miguel Ángel Collado-Corona4, Paul Shkurovich-Bialik4, Adalberto González-Astiazarán5. Localization of irritative zones in epilepsy with thermochromic silicone. 12-Jan-2022;13:14. Available from: https://surgicalneurologyint.com/surgicalint-articles/11338/
Abstract
Background: During epilepsy surgery, the gold standard to identify irritative zones (IZ) is electrocorticography (ECoG); however, new techniques are being developed to detect IZ in epilepsy surgery and in neurosurgery in general, such as infrared thermography mapping (ITM), and the use of thermosensitive/thermochromic materials.
Methods: In a cohort study of consecutive patients with focal drug-resistant epilepsy of the temporal lobe treated with surgery, we evaluated possible adverse effects to the transient placement of a thermochromic/thermosensitive silicone (TTS) on the cerebral cortex and their postoperative evolution. Furthermore, we compared the precision of TTS for detecting cortical IZ against the gold standard ECoG and with ITM, as proof of concept.
Results: We included 10 consecutive patients, 6 women (60%) and 4 men (40%). Age ranges from 15 to 56 years, mean 33.2 years. All were treated with unilateral temporal functional lobectomy. The mean hospital stay was 4 days. There were no immediate or late complications associated with the use of any of the modalities described. In the 10 patients, we obtained consistency in locating the IZ with ECoG, ITM, and the TTS.
Conclusion: The TTS demonstrated biosecurity in this series. The accuracy of the TTS to locate IZ was similar to that of ECoG and ITM in this study. More extensive studies are required to determine its sensitivity and specificity.
Keywords: Epilepsy surgery, Infrared thermography mapping, Irritative zone, Thermochromic silicone
INTRODUCTION
The effectiveness of epilepsy surgery depends on the location, resection, disconnection, or safe inhibition of the irritative zone (IZ). This is achieved through a meticulous clinical semiology, analysis of preoperative studies such as video electroencephalogram, magnetic resonance imaging, single-positron emission computed tomography, positron emission tomography (PET), or magnetoelectroencephalography. During epilepsy surgery, the gold standard to identify or corroborate the location of IZs is electrocorticography (ECoG); however, new techniques are being developed to detect those crucial areas in epilepsy surgery, such as infrared thermography mapping (ITM) and even more recently, using thermosensitive and thermochromic materials, which will be describen below.
The human brain has a high metabolic rate with high demands for glucose, oxygen, and blood flow and therefore with high heat production. There are thermal fluctuations during different physiological processes (generation of action potentials, resting potentials, synapses, neurotransmission, etc.) as well as in response to pathological processes such as tumors, cortical malformations, and infections.[
Infrared thermography in neurosurgery
IT cameras are a low-cost, remote, noninvasive, and real-time diagnostic tool capable of providing structural and qualitative functional information. Multiple studies have been carried out in the experimental field where this technique has been applied as an indicator of greater or lesser physiological activity.[
For the ITM to be reliable and reproducible, various factors that can generate temperature differences (ΔT) such as evaporation, differential thermal conduction, and altered surface physical phenomena (emissivity) must be taken into account, which are independent in each tissue, but it is recognized that water vapor is the best absorber of radiation.[
Some diagnostic techniques for IT currently in use are applied in infectious, inflammatory,[
It has been described that high-grade gliomas have a lower temperature than the surrounding healthy tissue due to a decrease in the metabolic rate due to edema and necrosis;[
Qualitative thermography and ITM open the possibility of offering the neurosurgeon a tool that gives an anatomical and physiological map with the potential to guide the resection of a lesion.[
There is a relationship between brain temperature, cerebral blood flow, focal brain activity, and the functional state of neurons[
In a study carried out in animals where a record was made with ECoG and correlation of the epileptogenic focus employing IT, an increase in thermal activity was found which was in contrast to the studies carried out by PET using fluorodeoxyglucose where the area epileptogenic was hypometabolic.[
Thermosensitive/thermochromic silicone (TTS)
Silicone is a widely used material in different settings of medical practice and its safety in neurosurgery has already been established.[
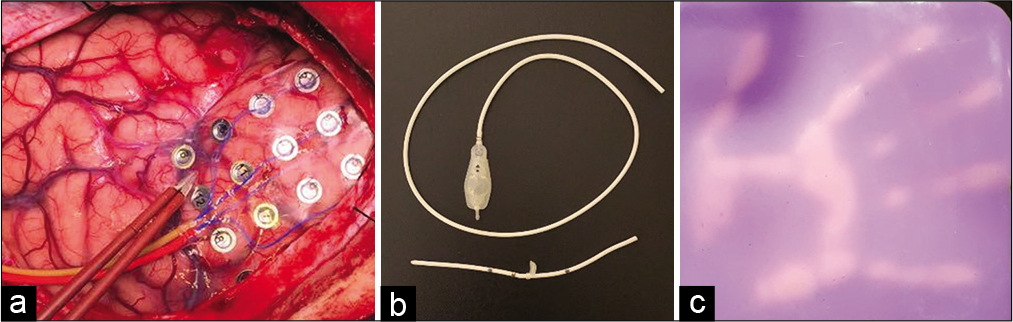
Figure 2:
Examples of silicone used in neurosurgery and heat-sensitive silicone. (a) Silicone grids routinely used in electrocorticography; (b) silicone ventriculoperitoneal shunt systems for permanent implantation routinely used in neurosurgery; and (c) change of color according to the temperature of the surface of the thermosensitive/thermochromic silicone. Its basal color at room temperature is purple and changes to pink when heated. Note the high spatiotemporal resolution of the material.
MATERIALS AND METHODS
With prior authorization from the ethics and research committees of our hospital and the signing of the informed consent of each patient, and according with the Helsinki Declaration of 1975, as revised in 2000, we included consecutive patients previously selected by the epilepsy clinic with a diagnosis of drug-resistant unilateral temporal focal epilepsy. We perform craniotomy following the protocol established for ITM,[
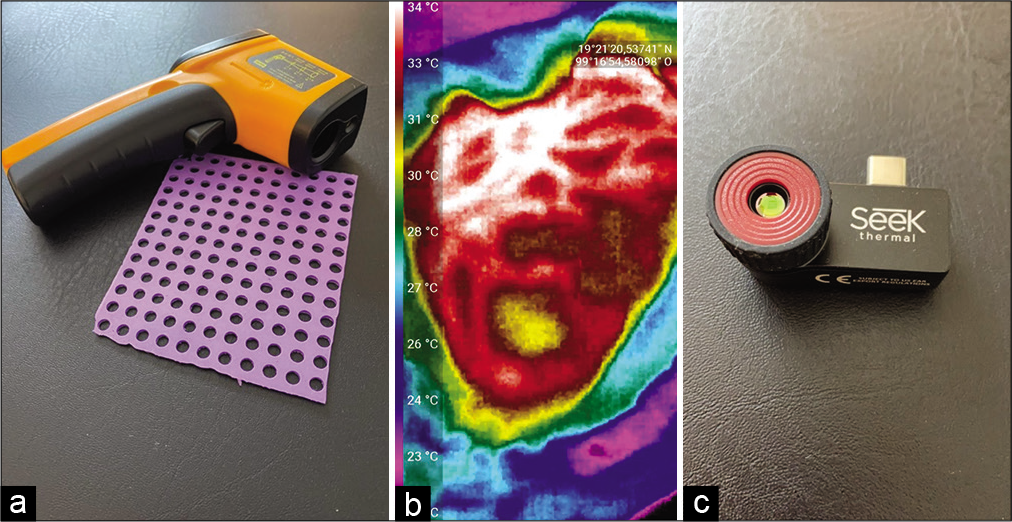
Figure 3:
(a) Laser pointer thermometer and silicone grid used in the first stage of the infrared thermography mapping (ITM), in which different sites are recorded through the holes in the silicone grid to establish a coordinate system during the first stage of the ITM. (b) Intraoperative image obtained by the high-resolution thermographic camera that allows obtaining photographs and videos used in the second stage of the ITM. The yellow color shows the irritative area in one of the cases of the series described in this article. (c) Thermography camera that connects to a cell phone and records high-resolution thermography videos and photos.
Figure 4:
Graphs of the measurements with the laser pointer thermometer of the first stage of the infrared thermography mapping. In this color code, the blue tones correspond to the coldest temperature registers and the red to the warmest ones. The presence of cold areas is noticeable, guarded by a radial heating pattern. All measurements are expressed in degrees centigrade. These cold areas coincide with the irritative areas detected by electrocorticography and with the hypothermic areas detected through the thermochromic/thermosensitive silicone in all the cases in this series. NV: Not assessable due to the presence of blood vessels.
The third stage of the evaluation of brain metabolism consists of placing the TTS on the brain surface. The TTS shows the observer a thermal imprint of the surface with which it is in contact in a period between 3 and 12 s. Its operating range is between 21 and 38°C. Its basal color is purple and when heated it changes to pink. The coldest areas remain in the basal purple color, allowing different thermal patterns to be identified with the naked eye. After a period of 2–40 s of being in contact with the surface of the brain, eventually, the entire plate of the TTS turns pink by induction of heat, so the reading of the thermal imprint should normally be done before the 20 s of placement. No special filter or light is required to observe the changes and they are easily perceived by any observer [
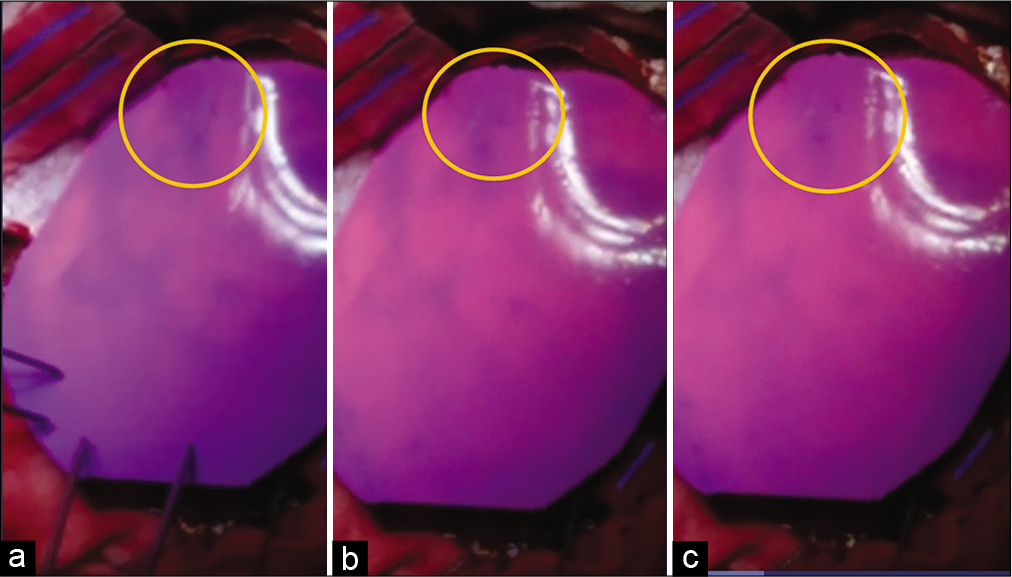
Figure 5:
Successive pictures of the changes in the color of the thermosensitive/thermochromic silicone when in contact with the brain surface. (a) At 2.50 s; (b) at 6.15 s; and (c) at 9.15 s. The yellow circle shows the irritative area detected by the electrocorticography. Note how the cooler irritant area remains in the purple basal color, for the silicone that begins to change rapidly (from ~3 s) to the pink color when heated by contact with the rest of the surface of the brain that has a normal temperature, around this zone of abnormal hypothermia.
RESULTS
Ten consecutive cases with surgical criteria for drug-resistant unilateral temporal focal epilepsy were included in our series. Of these, 6 were women (60%) and 4 men (40%). Age ranges from 15 to 56 years, mean 33.2 years. All patients were treated with unilateral functional temporal lobectomy. The mean hospital stay was 4 days. No intraoperative complications were observed and also during the 1st postoperative month. All patients without neurological morbidity were discharged. In all cases (100%), there was consistency in the location of the IZ between the ECoG, ITM, and TTS. All cases were diagnosed as III-A dysplasia according to the International League Against Epilepsy classification.
DISCUSSION
In neurological surgery, there is always a very delicate balance between the areas of the brain that must be preserved and those that must be removed. This can be even more difficult, if the appearance of healthy tissue is the same as that of diseased tissue to the naked eye, as occurs very frequently in epilepsy surgery since cortical dysplasias are the etiologies most frequently responsible for drug-resistant epilepsy and its gross appearance is indistinguishable from healthy brain tissue. To make surgeries more precise, effective, and safe, we have developed the ITM protocol[
In this series of 10 consecutive cases, there were no complications associated with the use of any of the methods described. There was a 100% correlation between ITM, TTS, and ECoG in detecting IZs in drug-resistant temporal lobe epilepsy.
CONCLUSION
The results described in this proof of concept are preliminary. Although we obtained consistency between the three methods to locate IZ in all the cases in this series, it is still necessary to carry out studies with a larger sample to determine its sensitivity and specificity, respecting the current experimentation guidelines in epilepsy surgery, in other neurosurgical etiologies, and in surgery of other organs and systems.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
The principal author participated in the design of the thermochromic silicone and may receive a fee if it is patented and commercialized.
References
1. de Font-Réaulx E, Lluch JT, López RL, Bialik PS, Corona MA, López LG. Thermography mapping patterns in temporal lobe epilepsy surgery. Surg Neurol Int. 2020. 11: 30
2. de Font-Réaulx E, López RL, López LG. Infrared thermography mapping plus neuronavigation target location in an eloquent area cavernoma resection. Surg Neurol Int. 2020. 11: 44
3. de Font-Réaulx Rojas E, Ochoa EE, López R, Díaz LG. Infrared thermography brain mapping surveillance in vascular neurosurgery for anterior communicating artery aneurysm clipping. Surg Neurol Int. 2018. 9: 188
4. Ecker RD, Goerss SJ, Meyer FB, Cohen-Gadol AA, Britton JW, Levine JA. Vision of the future: Initial experience with intraoperative real-time high-resolution dynamic infrared imaging. Technical note. J Neurosurg. 2002. 97: 1460-71
5. Elkins KC, Moncayo VM, Kim H, Olson LD. Utility of gray-matter segmentation of ictal-Interictal perfusion SPECT and interictal 18F-FDG-PET in medically refractory epilepsy. Epilepsy Res. 2017. 130: 93-100
6. Bondurant S, Ernster V, Herdman R.editors. Safety of Silicone Breast Implants. Washington, DC: National Academies Press; 1999. p.
7. Kateb B, Yamamoto V, Yu C, Grundfest W, Gruen JP. Infrared thermal imaging: A review of the literature and case report. Neuroimage. 2009. 47: T154-62
8. Kerr J, Zealand N. Review of the effectiveness of infrared thermal imaging (thermography) for population screening and diagnostic testing of breast cancer. Rev Lit Arts Am. 2004. 3: 1-49
9. King HH, Cayce CT, Herrin J. Thermography examination of abdominal area skin temperatures in individuals with and without focal-onset epilepsy. Explore (NY). 2017. 13: 46-52
10. Naydenov E, Minkin K, Penkov M, Nachev S, Stummer W. Infrared thermography in surgery of newly diagnosed glioblastoma multiforme: A technical case report. Case Rep Oncol. 2017. 10: 350-5
11. Salles MS, da Silva SC, Salles FA, Roma LC, El Faro L, Bustos Mac Lean PA. Mapping the body surface temperature of cattle by infrared thermography. J Therm Biol. 2016. 62: 63-9
12. Saxena AK, Willital GH. Infrared thermography: Experience from a decade of pediatric imaging. Eur J Pediatr. 2008. 167: 757-64
13. Steiner G, Sobottka SB, Koch E, Schackert G, Kirsch M. Intraoperative imaging of cortical cerebral perfusion by time-resolved thermography and multivariate data analysis. J Biomed Opt. 2011. 16: 16001
14. Suárez-Piñera M, Mestre-Fusco A, Ley M, González S, Medrano S, Principe A. Perfusion SPECT, SISCOM and 18-FDG in the evluation of drug resistant epileptic patients who are candidates for epilepsy surgery. Rev Esp Med Nucl Mol Image. 2015. 34: 350-7
15. Sunderam S, Osorio I. Mesial temporal lobe seizures may activate thermoregulatory mechanisms in humans: An infrared study of facial temperature. Epilepsy Behav. 2003. 4: 399-406
16. Tattersall GJ. Infrared thermography: A non-invasive window into thermal physiology. Comp Biochem Physiol A Mol Integr Physiol. 2016. 202: 78-98
17. Wang H, Wang B, Normoyle KP, Jackson K, Spitler K, Sharrock M. Brain temperature and its fundamental properties: A review for clinical neuroscientists. Front Neurosci. 2014. 8: 307
18. Wintermark P, Lechpammer M, Warfield SK, Kosaras B, Takeoka M, Poduri A. Perfusion imaging of focal cortical dysplasia using arterial spin labeling: Correlation with histopathological vascular density. J Child Neurol. 2013. 28: 1474-82