- Department of Neurosurgery, Hospital Hernan Henriquez Aravena, Temuco, Chile
- Department of Neurosurgery, Hospital Regional de Coyhaique, Coyhaique, Aysen, Chile,
- Department of Internal Medicine, Hospital Militar Coronel Elbano Paredes Vivas, Maracay, Venezuela,
- Department of General Medicine, CESFAM El Aguilucho, Santiago de Chile, Chile,
- Department of Neurosurgery, Hospital Vargas of Caracas, San José, Caracas,
- Department of Neurosurgery, Clinica Chilemex, Ciudad Guayana, Venezuela
- Department of Neurosurgery, Centro Medico Docente La Trinidad, Baruta, Venezuela,
- Department of Neuroscience, CEA LETI CLINATEC, Grenoble, France,
- Department of Neuro-diagnostics, Neurology Mobile System Associates, Miami, Florida, United States.
Correspondence Address:
Sergio Antonio Sacchettoni, Department of Neuro-diagnostics, Neurology Mobile System Associates, Miami, Florida, United States.
DOI:10.25259/SNI_673_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Darelys Teresa Lopez1, Gabriel E. Manzano2, Asveth Medina3, Maria Jose Prieto4, Juan Pointcarré Abud5, Luis Salazar6, Maria Fernanda Vargas7, Napoleon Torres8, Sergio Antonio Sacchettoni9. Long-term follow-up of Parkinsonian patients operated on with deep brain electromodulation without intraoperative microrecording. 22-Dec-2023;14:435
How to cite this URL: Darelys Teresa Lopez1, Gabriel E. Manzano2, Asveth Medina3, Maria Jose Prieto4, Juan Pointcarré Abud5, Luis Salazar6, Maria Fernanda Vargas7, Napoleon Torres8, Sergio Antonio Sacchettoni9. Long-term follow-up of Parkinsonian patients operated on with deep brain electromodulation without intraoperative microrecording. 22-Dec-2023;14:435. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=12686
Abstract
Background: Deep brain electromodulation (DBEM), also known as deep brain stimulation in different intracerebral targets, is the most widely used surgical treatment due to its effects in reducing motor symptoms of Parkinson’s disease. The intracerebral microelectrode recording has been considered for decades as a necessary tool for the success of Parkinson’s surgery. However, some publications give more importance to intracerebral stimulation as a better predictive test. Since 2002, we initiated a technique of brain implant of electrodes without micro recording and based solely on image-guided stereotaxis followed by intraoperative macrostimulation. In this work, we analyze our long-term results, taking into account motor skills and quality of life (QL) before and after surgery, and we also establish the patient’s time of clinical improvement.
Methods: This is a descriptive clinical study in which the motor state of the patients was evaluated with the unified Parkinson’s disease scale (UPDRS) and the QL using the Parkinson’s disease QL questionnaire 39 questionnaires before surgery, in the “on” state of the medication; and after surgery, under active stimulation and in the “on” state.
Results: Twenty-four patients with ages ranging from 37 to 78 years undergoing surgery DBEM on the subthalamic nucleus were studied. An improvement of 41.4% in motor skills and 41.7% in QL was obtained.
Conclusion: When microrecording is not available, the results that can be obtained, based on preoperative imaging and clinical intraoperative findings, are optimal and beneficial for patients.
Keywords: Brain microrecording, Deep brain electromodulation, Deep brain stimulation, Image-guided implantation, Parkinson’s disease, Parkinson’s surgery, Quality of life
INTRODUCTION
Deep brain electromodulation (DBEM), also known as “deep brain stimulation,” is a surgical approach that offers multiple benefits for the symptoms of Parkinson’s disease (PD), especially using the subthalamic nucleus (STN) as the target.[
MATERIALS AND METHODS
This is a clinical study with a descriptive design, in which a series of cases is analyzed to evaluate the effectiveness of DBEM, including the duration of improvement after surgery. The data were obtained directly from patients who underwent surgery from January 2009 to December 2017 in three different centers, all operated on by the same surgeon (SAS).
Patients had a diagnosis of PD of 5 years or more, of both sexes, aged between 40 and 80 years. The response to L-dopa (RL) was reported as a difference in the score (OFF-ON; absolute LR)[
All the patients were informed about the purpose of the study, they were guaranteed complete confidentiality of the data obtained, and they gave their consent for inclusion in the study.
The surgical technique was already described elsewhere.[
Of 32 patients who underwent surgery, six could not be contacted to obtain a follow-up. One had the electrodes removed because he developed rejection and infection of the surgical scar (see complications in the Result and Analysis chapter). One did not consent to participate in the study. In total, our study is based on 24 patients.
The variables studied in each patient were motor skills and quality of life (QL). Motor skills were assessed using the motor UPDRS scale[
QL was assessed through the Parkinson’s disease QL questionnaire (PDQ-39).[
Finally, an item called “subjective quality of life” was included, in which the patient was asked, “On a scale of 1–10, how do you feel about your QL?” both before and after the operation, thus evaluating how the patient feels according to their criteria.
The database was made using the Excel 2003® software, and for the data analysis, the EpiInfo 3.5.433® software. To compare the means before and after surgery of the motor values (motor UPDRS), QL (PDQ-39), and subjective QL, the paired Student’s t-test was used, considering P < 0.0001 as significant in all cases.
Each variable was recorded at two times: Before surgery, in the “on” state, and after surgery, in the “on” state as well, and with the optimal programming of the DBEM system.
Follow-up was from 5 to 14 years, mean of 7.89 years.
RESULTS
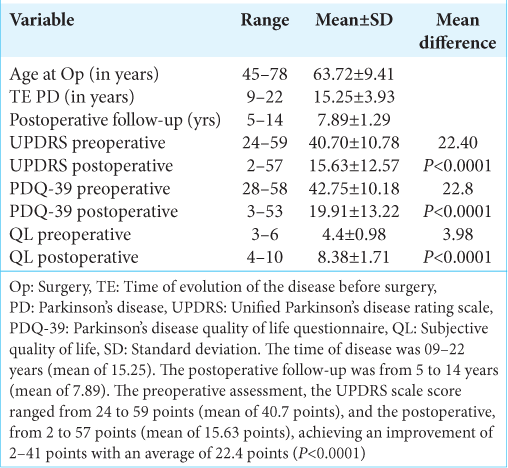
Eleven (11) patients were women (45.83%), and 13 were men (54.17%), with ages ranging from 45 to 78 years (mean of 63.72) at the time of surgery [
The time of disease was 09–22 years (mean of 15.25). The postoperative follow-up was from 5 to 14 years (mean of 7.89) [
In the preoperative assessment, the motor UPDRS scale score ranged from 24 to 59 points (mean of 40.7 points), and in the postoperative, from 2 to 57 points (mean of 15.63 points), achieving an improvement of 2–41 points with an average of 22.4 points (P < 0.0001) [
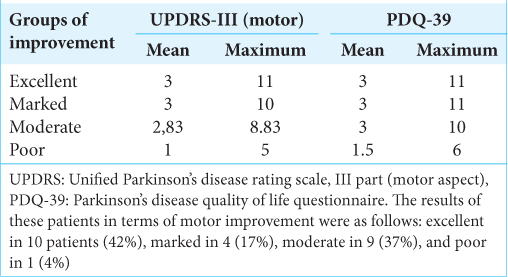
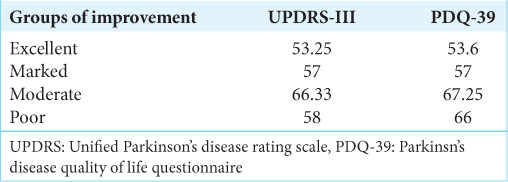
The results of these patients in terms of motor improvement were as follows: excellent in 10 patients (42%), marked in 4 (17%), moderate in 9 (37%), and poor in 1 (4%).
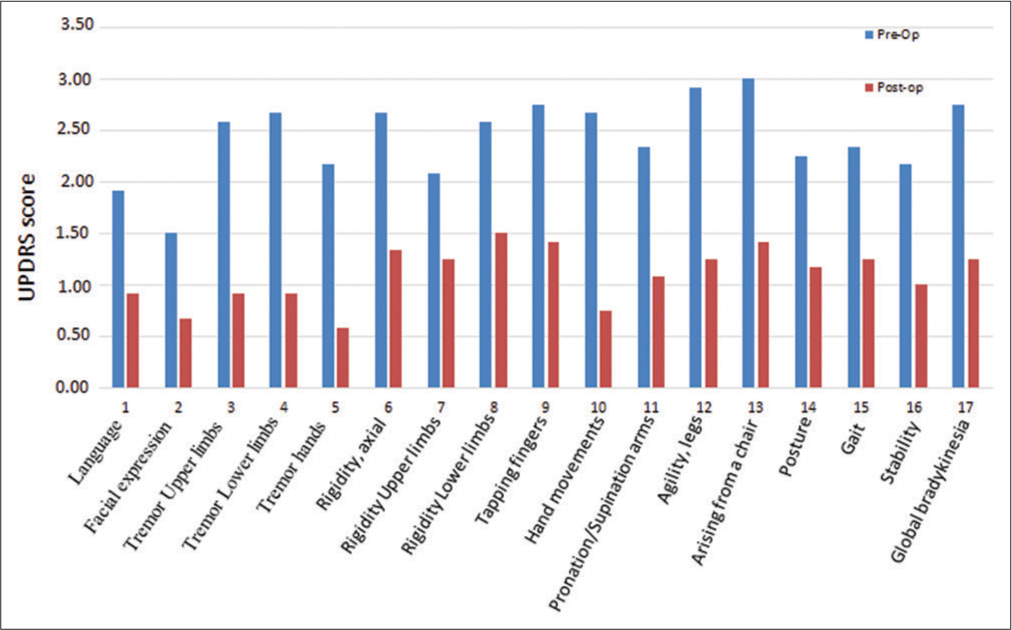
Preoperatively, the highest scores in motor parameters were as follows: standing up from a chair (“arising from a chair,” mean 3 points; standard deviation [SD]: 0.95), agility in the lower limbs (mean 2.92 points; SD: 0.9), movement (bradykinesia) and finger tapping (both mean 2.75 points; SD: 0.8 and 1.42, respectively), axial rigidity, alternating movements (“pronation/supination arms”), and tremor in the lower limbs (average 2.67 points; SD 0.98, 1.07, 1.15, respectively). A significant reduction in their score was subsequently achieved postoperatively, between 48.4% and 71.8% [
Graph 1:
Comparison of the mean of unified Parkinson’s disease rating scale (UPDRS) score on motor symptoms before and after surgery. We can observe that all of them were reduced (improved). Preoperatively, the highest scores in motor parameters were as follows: standing up from a chair (“arising from a chair,” mean 3 points; standard deviation [SD]: 0.95), agility in the lower limbs (mean 2.92 points; SD: 0.9), movement (bradykinesia) and finger tapping (both mean 2.75 points; SD: 0.8 and 1.42, respectively), axial rigidity, alternating movements (“pronation/supination arms”), and tremor in the lower limbs (average 2.67 points; SD 0.98, 1.07, 1.15, respectively). Postoperatively, a significant reduction in their score was subsequently achieved between 48.4% and 71.8%. The motor parameters that had the highest percentage of postoperative improvement were as follows: hand tremor (73.0%), alternating movements with the hands (“pronation/supination arms,” 71.8%), and leg (65.6%) and arm (64,5%) tremor. The axial symptoms that were taken into account were Language, standing up from a chair (“arising from a chair”), posture, postural stability (“stability”), and gait. A preoperative score was obtained for these items, with a mean of 11.67 points (SD: 3.7) and a postoperative score of 5.75 points (SD: 5.81), achieving an average reduction (improvement) of 5.92 points (SD: 4.6), which represents a 50.71% improvement in axial symptoms after surgery.
The motor parameters that had the highest percentage of postoperative improvement were as follows: hand tremor (73.0%), alternating movements with the hands (“pronation/supination arms,” 71.8%), and leg (65.6%) and arm (64,5%) tremor [
The axial symptoms that were taken into account were as follows: Language, standing up from a chair (“arising from a chair”), posture, postural stability (“stability”), and gait. A preoperative score was obtained for these items, with a mean of 11.67 points (SD: 3.7) and a postoperative score of 5.75 points (SD: 5.81), achieving an average reduction (improvement) of 5.92 points (SD: 4.6), which represents a 50.71% improvement in axial symptoms after surgery.
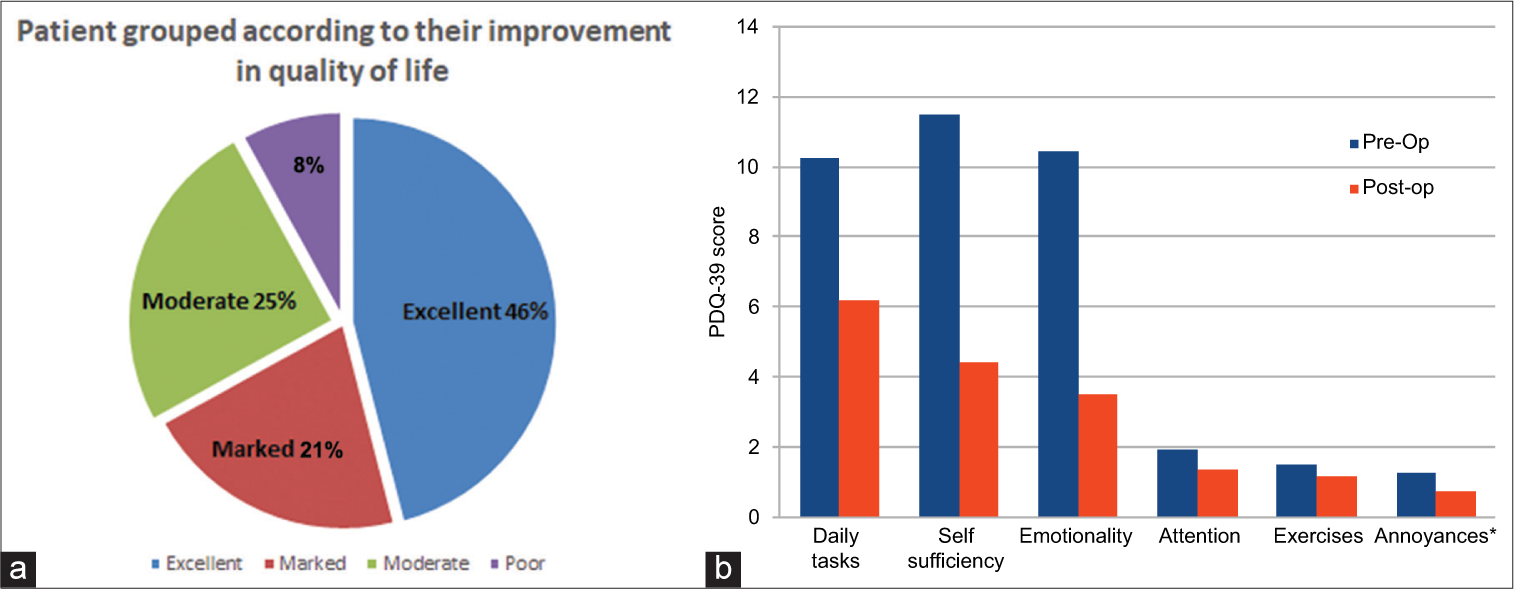
Regarding the analysis of QL, the preoperative PDQ-39 score ranged from 28 to 58 points (mean 42.75 points), and the postoperative was 3 to 53 points (mean 19, 91 points); thus, the average improvement was 22.8 points. The results of these patients in terms of improvement in QL were as follows: Excellent improvement in 11 patients (46%), marked improvement in 5 (21%), moderate improvement in 6 (25%), and poor improvement in 2 individuals (8%) [
Graph 2:
(a) Patients grouped according to their improvement in quality of life (QL). Regarding the analysis of QL, the preoperative Parkinson’s disease QL questionnaire-39 score ranged from 28 to 58 points (mean 42.75 points), and the postoperative was 3–53 points (mean 19, 91 points); thus, the average improvement was 22.8 points. The results of these patients in terms of improvement in QL were as follows: Excellent improvement in 11 patients (46%), marked improvement in 5 (21%), moderate improvement in 6 (25%), and poor improvement in 2 individuals (8%). (b) Patients were grouped according to their improvement in QL. Comparison of the mean QL aspects before and after surgery (*annoyances: Painful muscles rigidity, pain in joints). The characteristics of the QL that had the greatest improvement were emotionality (in 66.40%), self-sufficiency (in 61.59%), and the performance of daily tasks (in 39.84%).
In the subjective evaluation of QL, where 10 is the best score as QL according to the personal opinion of the patient, we found a preoperative score that ranged from 3 to 6 points (mean 4.5 points) and postoperative from 4 to 10 points (average 7.41 points) with an average improvement of 2.91 points.
Regarding the time of postoperative motor improvement, it varies among the patients, and it was compared with the degree of improvement. In
When relating the patient’s age at the time of surgery to the motor improvement and QL groups, we did not observe a significant difference [
As mentioned above, some patients had complications and were not included in this series. One of them had an infection beneath one of the burr-hole covers, which secures the lead. When we reoperated, the pus was extending deeper, so we had to remove that side of the whole system. After three months under antibiotics, we repositioned a new lead. The patient is recovering from her previous improvement. Another complication was a rupture of the intra-cerebral portion of one lead. We had to change it for a new one. Finally, another one consisted of an upward displacement of the lead, probably due to not having been properly secured with the burr-hole cover. We replaced it and properly secured it.
DISCUSSION
This study highlights the benefit of DBEM based on image-guided stereotaxys followed by intraoperative macrostimulation in PD patients who met the inclusion criteria used in this work. Similar to other studies,[
The motor aspect was clearly the symptom that improved the most, showing an improvement in the motor UPDRS scores of approximately 48% attributable to the effect of the DBEM combined with pharmacological therapy (“on” + DBEM), compared to the initial motor state before surgery, under the effects of exclusive medication (“on”), and there was a significant difference. These results are similar to those shown in other publications using MER.[
Furthermore, patients with the best motor response to DBEM (42%) showed a better motor state after three years after surgery. These patients were between 45 and 57 years old at the time of surgery and the shortest time of the disease. However, these ages at the time of surgery did not show a statistical difference compared with older ages in our series. The rest of the patients showed a progressive decrease in improvement in the same follow-up time, had a longer time of disease, and had an older age, both at the time of surgery.
An improvement in axial symptoms was observed in 50.71% of this study but did not last as long as the global motor skills improvement, assessed by the motor UPDRS scale. In another study, an improvement in axial symptoms has been reported as much as 45.4%.[
In regard to the QL, the aspects that showed the best improvement were emotionality (66.40%), self-sufficiency (61.59%), and performance of daily tasks (39.84%), which is also equivalent to other publications.[
Regarding the subjective self-assessment of the QL, we also found a postoperative average improvement of 2.91 points. These results are similar to those shown in other publications using MER.[
The improvement of motor and neurobehavioral symptoms translates into results that have a favorable impact on improving the QL of patients.[
The present sample is small, and a larger series, with full follow-up, is necessary to determine the impact of DBEM using an image-guided stereotaxys followed by intraoperative macrostimulation in PD.
The use of MER goes back to the time when imaging was imprecise (i.e., ventriculography). Thus, adding MER increased the probability of reaching the target.[
CONCLUSION
In conclusion, DBEM based solely on preOp imaging and clinical intraoperative findings is a treatment that offers as good results as those published with MER. In fact, there is now a tendency to use imaging as the only reference to locate the target, the STN.[
Ethical approval
Approved by the Institutional Ethics Committee (IEC), letter HV20080612 dated 06/12/2008.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgments
Dr. Luz Marina Navarrete, for reviewing the methodology. Prof. Carlos Espino, for statistical analysis. Every patient who was keen to cooperate with this study. Training in Parkinson’s surgery of one of the authors (SAS) was under Professors Rafael Galera and Juan Felix Del Corral from Hospital Vargas de Caracas, Venezuela, and Patrick Mertens and Marc Guenot from Hôpital Neurologique de Lyon, France. This work is dedicated to them.
References
1. Abud JP, Mantilla P, Pinero A, Galue R, Del Corral JF, Sacchettoni S. Implant of electrodes for deep brain electromodulation of the subthalamic nucleus in Parkinson’s disease without intraoperative microrecording (Spanish). Neurotarget. 2009. 4: 59-65
2. Bertrand G, Jasper H, Wong A, Mathews G. Microelectrode recording during stereotactic surgery. Clin Neurosurg. 1969. 16: 328-55
3. Benabid AL, Chabardes S, Mitrofanis J, Pollak P. Deep brain stimulation of the subthalamic nucleus for the treatment of Parkinson’s disease. Lancet Neurol. 2009. 8: 67-81
4. Benazzouz A, Breit S, Koudsie A, Pollak P, Krack P, Benabid AL. Intraoperative microrecordings of the subthalamic nucleus in Parkinson’s disease. Mov Disord. 2002. 17: S145-9
5. Caire F, Ranoux D, Guehl D, Burbaud P, Cuny E. A systemic review of studies on anatomical position of electrode contacts used for chronic subthalamic stimulation in Parkinson’s disease. Acta Neurochir. 2013. 155: 1647-54
6. Collomb-Clerc A, Welter ML. Effects of deep brain stimulation on balance and gait in patients with Parkinson’s disease: A systematic neurophysiological review. Neurophysiol Clin. 2015. 45: 371-88
7. Follet KA, Torres-Russotto D. Deep brain stimulation of globus pallidus interna, subthalamic nucleus, and pedunculopontine nucleus for Parkinson’s disease: Which target?. Parkinsonism Relat Disord. 2012. 18: S165-7
8. Ford B, Winfield L, Pullman S, Frucht S, Du Y, Greene P. Subthalamic nucleus stimulation in advanced Parkinson’s disease: Blinded assessments at one year follow up. J Neurol Neurosurg Psychiatry. 2004. 75: 1255-9
9. Gildenberg PL, Lunsford LD, editors. General concepts of stereotactic surgery. Modern stereotactic neurosurgery. Boston: Martinus Nijhoff; 1988. p. 3-12
10. Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P. Movement disorder society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov Disord. 2008. 23: 2129-70
11. Hamel W, Fietzek U, Morsnowski A, Schrader B, Weinert D, Müller D. Subthalamic nucleus stimulation in Parkinson’s disease: Correlation of active electrode contacts with intraoperative microrecording. Stereotact Funct Neurosurg. 2003. 80: 37-42
12. Malinova V, Pinter A, Dragaescu C, Rohde V, Trenkwalder C, Sixel-Döring F. The role of intraoperative microelectrode recording and stimulation in subthalamic lead placement for Parkinson’s disease. PLoS One. 2020. 15: e0241752
13. Monteiro A, Andrade C, Rosas M, Linhares P, Massano J, Vaz R. Deep brain stimulation of the subthalamic nucleus in advanced Parkinson’s disease. Follow-up for 5 years in a portuguese center. Rev Neurol. 2014. 58: 433-40
14. Mosley PE, Marsh R. The psychiatric and neuropsychiatric symptoms after subthalamic stimulation for Parkinson’ disease. J Neuropsychiatry Clin Neurosci. 2015. 27: 19-26
15. Odekerken VJ, van Laar T, Staal MJ, Mosch A, Hoffman CF, Nijssen PC. Subthalamic nucleus versus globus pallidus bilateral deep brain stimulation for advanced Parkinson’s disease (NSTAPS study): A randomized controlled trial. Lancet Neurol. 2013. 12: 37-44
16. Pollak P, Krack P, Fraix V, Mendes A, Moro E, Chabardes S. Intraoperative micro-and macrostimulation of the subthalamic nucleus in Parkinson’s disease. Mov Disord. 2002. 17: S155-61
17. Pieterman M, Adams S, Jog M. Method of levodopa response calculation determines strength of association with clinical factors in Parkinson disease. Front Neurol. 2018. 9: 260
18. Rodriguez M, Zamarbide I, Guridi J, Palmero M, Obeso J. Efficacy of deep brain stimulation of the subthalamic nucleus in Parkinson’s disease 4 years after surgery: Double blind and open label evaluation. J Neurol Neurosurg Psychiatry. 2004. 75: 1382-5
19. Ruiz-Garcíaa MV, Gómez-Hontanillaa M, Ruiz-Garcíab AM, Ruiz-Garcíac J, Ruiz-Garcíad A, Herráez-Izquierdoa V. Quality of life of parkinson’s patients after surgical treatment at the university hospital complex of albacete. Rev Cient Soc Españ Enferm Neurol. 2011. 33: 10-5
20. Sacchettoni SA, Del Corral JF, Abud JP. Deep brain electro-stimulation for Parkinson´s disease in the subthalamic nucleus without micro-recording (Spanish). Informe Médico. 1009. 11: 165-168
21. Savas A, Akbostanci C, Yagmurlu B, Elibol B, Erden I, Kanpolat Y. A new method for subthalamic nucleus targeting using CT/MRI image-fusion technology. Acta Neurochir. 2002. 144: 1076-7
22. Volonté MA, Clarizio G, Galantucci S, Scamarcia PG, Cardamone R, Barzaghi LR. Long term follow-up in advance Parkinson’s disease treated with DBS of the subthalamic nucelus. J Neurol. 2021. 268: 2821-30
23. Wang J, Ponce FA, Tao J, Yu HM, Liu JY, Wang YJ. Comparison of awake and asleep deep brain stimulation for Parkinson’s disease: A detailed analysis through literature review. Neuromodulation. 2020. 23: 444-50