- Departments of Anesthesiology, Naresuan University, Tambon Thapho, Muang Phitsanulok, Thailand.
- Departments of Surgery, Naresuan University, Tambon Thapho, Muang Phitsanulok, Thailand.
Correspondence Address:
Patcharin Intarakhao
Departments of Anesthesiology, Naresuan University, Tambon Thapho, Muang Phitsanulok, Thailand.
DOI:10.25259/SNI_79_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Patcharin Intarakhao1, Peeraphong Thiarawat2, Apirak Tewaritrueangsri1, Surachart Pojanasupawun1. Low-dose adenosine-induced transient asystole during intracranial aneurysm surgery. 08-Aug-2020;11:235
How to cite this URL: Patcharin Intarakhao1, Peeraphong Thiarawat2, Apirak Tewaritrueangsri1, Surachart Pojanasupawun1. Low-dose adenosine-induced transient asystole during intracranial aneurysm surgery. 08-Aug-2020;11:235. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=10191
Abstract
Background: Few studies have evaluated the adenosine dose that induces cardiac arrest during intracranial aneurysm surgery. We present our experiences with adenosine-induced transient asystole (AiTA) during intracranial aneurysm surgery and dosage recommendations.
Methods: We retrospectively reviewed the medical records of all patients who underwent intracranial aneurysm surgery between July 2016 and December 2018. Patients who experienced AiTA during intracranial aneurysm surgery were included in the study.
Results: Our study included nine intracranial aneurysm surgeries performed in eight patients. Thirteen episodes of AiTA were reported. Five of these were performed to facilitate bleeding control due to intraoperative aneurysm rupture (IAR), and adenosine doses were 9 mg (0.20 mg/kg), 12 mg (0.25 mg/kg), 12 mg (0.26 mg/kg), 18 mg (0.34 mg/kg), and 18 mg (0.39 mg/kg), resulted in transient asystole for 12, 14, 9, 44, and 18 s, respectively. For episodes without IAR, adenosine doses ranging from 6 to 18 mg (0.11–0.39 mg/kg) caused asystole for 8–33 s. In five episodes without IAR, low-dose adenosine (lower than 0.2 mg/kg) was used and caused asystole ranging from 8 to 12 s. Postoperatively, two patients had elevated cardiac troponin T levels but normal electrocardiograms.
Conclusion: AiTA can facilitate the clipping of intracranial aneurysms at low-risk of serious cardiac complications. An adenosine dose of 0.2–0.4 mg/kg is safe and effective in both IAR and non IAR situations. In non IAR cases, we propose that low-dose AiTA is an option to facilitate aneurysm clipping. A starting dose of 6 mg or 0.1–0.2 mg/kg can adequately induce brief asystole by softening the aneurysmal sac during clip application.
Keywords: Adenosine, Cerebral aneurysm, Induced transient asystole, Subarachnoid hemorrhage, Surgery
INTRODUCTION
During the intraoperative aneurysm rupture (IAR), the proximal control and cardiac standstill have been used to facilitate bleeding control. Several transient cardiac standstill techniques have been evolved including deep hypothermia with circulatory arrest on cardiopulmonary bypass, rapid ventricular pacing, and intraoperative adenosine-induced transient asystole (AiTA).
AiTA was proved to be an effective and safe technique to minimize bleeding caused by IAR. Recently, AiTA was shown to be useful as an alternative technique for the decompression of aneurysms during clip application. Several studies have demonstrated the utility of AiTA in facilitating intracranial aneurysm clip ligation and its safety regarding perioperative and postoperative outcomes.[
The previous studies have used various starting doses of adenosine. However, few have explored its optimal dosage. We present a case series of using AiTA and determine the appropriate dose of adenosine for IAR and without IAR (non IAR) situations during intracranial aneurysm surgery.
MATERIALS AND METHODS
This study was approved by our institutional review board. We retrospectively reviewed the medical records, radiographic characteristics, anesthetic records, and operative records of all patients who underwent intracranial aneurysm surgery between July 2016 and December 2018. Patients who received AiTA were included in the study.
The anesthesiologists performed general anesthesia. The anesthetic management included arterial catheterization and standard monitoring, and external pacing-defibrillator pads were prepared. The patient was inducted with fentanyl and propofol, and cisatracurium was used to facilitate endotracheal intubation. Anesthesia was maintained with a propofol infusion and/or inhaled sevoflurane (<0.5 minimum alveolar concentration) and a fentanyl bolus. A bolus dose of IV nicardipine was used to control hypertension. The adenosine was administered in an antecubital vein as an intravenous bolus followed by a rapid saline infusion. Repeat doses were administered after circulation returned to baseline values. The decision to use adenosine was made by the surgeon.
The following data were collected: sex, age, comorbidities, height, weight, aneurysm location, size, rupture status, dose of adenosine, reason for adenosine use, hemodynamics after the adenosine bolus, success of the clipping facilitated by AiTA, surgeon satisfaction, and postoperative complications. For cardiovascular complications, the electrocardiograms (EKGs) and cardiac enzymes were reviewed.
The descriptive data are presented as the mean ± SD for normally distributed data, as the median (range) for continuous variables, and as the count (percentage) for categorical variables.
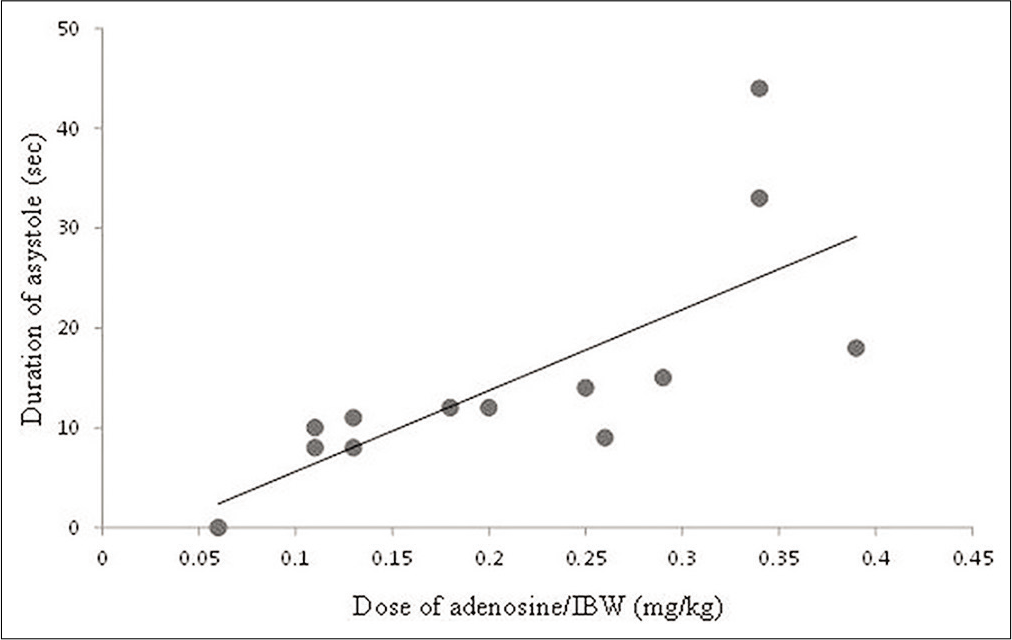
For each patient, a scattergram of the adenosine dose and the duration of asystole was constructed. The relationship between the dose of adenosine (mg per ideal body weight [IBW] in kg) and the duration of asystole was examined by a linear regression analysis.
RESULTS
We identified 26 patients who underwent 28 intracranial aneurysm surgeries in our institutions between July 2016 and December 2018. Of these 26 patients, eight who underwent nine intracranial aneurysm surgeries experienced 13 episodes of AiTA. These patients included six females and two males with a mean age of 61.6 ± 5.4 (range 55–72) years. The patient data and aneurysm characteristics are summarized in [
The details of the patients and episodes of AiTA are shown in [
The majority of cases were emergency surgery due to ruptured aneurysms. The patient #3 harboring bilateral Pcom aneurysms presented with a subarachnoid hemorrhage. We performed two separate clipping surgeries. The first clipping was for the ruptured side, while the second operation was an elective clipping for the contralateral unruptured aneurysm.
Of these 13 episodes of AiTA, five episodes of AiTA were used during an IAR to facilitate bleeding control. The other eight episodes of AiTA without IAR (Non IAR) were used as an alternative to temporary clipping.
The dose of adenosine ranged from 3 to 18 mg (0.06–0.39 mg/kg IBW). The duration of asystole ranged from 0 to 44 s. Four patients required a second dose of adenosine (patient #1, #4, #6, and #7), one for further dissection or clip adjustment (patient #6), and three to facilitate bleeding control due to IAR (patient #1, #4, and #7). One patient with an adenosine dose of 3 mg (0.06 mg/kg IBW) did not achieve asystole. However, the systolic blood pressure decreased from baseline for 10 s, thereby allowing for a successful aneurysm clipping. Intraoperative ruptures did not occur in any case during adenosine-induced flow arrest.
For those with IAR (patient #1, #3, #4, and #7), the dose of adenosine was 9, 12, 12, 18, and 18 mg (0.20–0.39 mg/kg IBW), and the duration of asystole was 12, 9, 14, 18, and 44 s, respectively.
Low-dose adenosine (lower than 0.2 mg/kg) was used in six episodes of non IAR. Adenosine applied at 3 mg did not induce asystole, whereas four episodes treated with 6 mg of adenosine (0.11–0.13 mg/kg) and one episode treated with 12 mg (0.18 mg/kg) caused asystole for 8–12 s. Clip application was successfully performed, and additional doses of adenosine were not required. Low- dose AiTA was used in one episode to control bleeding during IAR; in this case, adenosine (9 mg, 0.2 mg/kg) induced 12 s of asystole.
Scattergrams of the adenosine dose (mg/kg IBW) and the duration of asystole (s) are shown in [
Postoperatively, two patients had elevated cardiac troponin T levels, but none of their EKGs showed any changes. Atrial fibrillation (AF) occurred after adenosine dosing in one patient who had preoperative PACs. Amiodarone was initiated due to the degree of hypotension in that patient. However, the patient’s postoperative cardiac enzyme level was normal, and the AF changed to a sinus rhythm in the intensive care unit. In the other patients, the degree of hypotension or bradycardia resulting from AiTA was transient and resolved to normal baseline values.
DISCUSSION
During intracranial aneurysm surgery, AiTA was initially used in cases where temporary clipping was infeasible and to facilitate bleeding control if IAR occurred.[
Many studies have demonstrated the safety of this technique with regard to perioperative cardiac morbidity.[
At present, there are two techniques for adenosine administration in AiTA, with each requiring different dosages of adenosine: the dose-escalation technique and the dose estimation technique. In the dose-escalation technique, 6–12 mg of adenosine is initially used, and then more doses are added in a titrated manner. In the dose estimation technique, a single dose of adenosine is administered to achieve a predictable asystolic time. In dose-response studies, dosages ranging from 0.24 to 0.42 mg/kg IBW were recommended to achieve 30–60 s of profound hypotension and bradycardia.[
In cases of IAR, it is useful to maintain asystole until temporary clips can be placed on the parent vessels or permanent clips can be placed on the aneurysm’s neck. Thus, using AiTA might require a long asystolic duration. The findings of this study support the dosages of adenosine reported in previous studies.[
The purpose of using AiTA in non IAR cases is to prevent IAR during permanent clip application and minimize blood loss if IAR occurs. Intarakhao et al.[
In our study, one patient who had preoperative PACs developed AF after adenosine dosing. Pre-existing cardiac conduction abnormalities might have been the cause of this patient’s cardiac arrhythmia, and the cardiac asystole was also longer in this patient than that observed in other patients. Therefore, clinicians should be aware of whether the patient has any conditions including severe reactive airway disease, severe coronary artery disease, and pre-existing cardiac connection abnormalities[
The previous studies[
Regarding the study limitations, the study design was retrospective, and it included a relatively small number of patients. In addition, the duration of asystole was recorded manually. The details about the duration of hypotension and precise heart rate after the bolus of adenosine are lacking, which might have been beneficial in our study. Finally, each surgeon needs a different working time, so the request for a repeat dose of adenosine might be dependent on the individual.
CONCLUSION
AiTA can facilitate the clipping of intracranial aneurysms and has a low incidence of serious complications. When using AiTA during IAR, we suggest using an estimated dose of 0.2–0.4 mg/kg to provide long asystole to facilitate bleeding control and that additional doses of adenosine should be minimized. Under non IAR conditions, a low dose of adenosine (6 mg or 0.1–0.2 mg/kg) can be considered as an option to facilitate clip application. However, further studies with larger numbers of patients are needed to refine our understanding of the use of adenosine during aneurysm surgery.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Baker AB, Bookallil MJ, Lloyd G. Intentional asystole during endoluminal thoracic aortic surgery without cardiopulmonary bypass. Br J Anaesth. 1997. 78: 444-8
2. Bebawy JF, Gupta DK, Bendok BR, Hemmer LB, Zeeni C, Avram MJ. Adenosine-induced flow arrest to facilitate intracranial aneurysm clip ligation: Dose-response data and safety profile. Anesth Analg. 2010. 110: 1406-11
3. Bebawy JF, Zeeni C, Sharma S, Kim ES, DeWood MS, Hemmer LB. Adenosine-induced flow arrest to facilitate intracranial aneurysm clip ligation does not worsen neurologic outcome. Anesth Analg. 2013. 117: 1205-10
4. Belloni FL, Wang J, Hintze TH. Adenosine causes bradycardia in pacing-induced cardiac failure. Circulation. 1992. 85: 1118-24
5. Bendok BR, Gupta DK, Rahme RJ, Eddleman CS, Adel JG, Sherma AK. Adenosine for temporary flow arrest during intracranial aneurysm surgery: A single-center retrospective review. Neurosurgery. 2011. 69: 815-20
6. Blardi P, Urso R, De Lalla A, Volpi L, Perri TD, Auteri A. Nimodipine: Drug pharmacokinetics and plasma adenosine levels in patients affected by cerebral ischemia. Clin Pharmacol Ther. 2002. 72: 556-61
7. Desai VR, Rosas AL, Britz GW. Adenosine to facilitate the clipping of cerebral aneurysms: Literature review. Stroke Vasc Neurol. 2017. 2: 204-9
8. Groff MW, Adams DC, Kahn RA, Kumbar UM, Yang BY, Bederson JB. Adenosine-induced transient asystole for management of a basilar artery aneurysm. Case report. J Neurosurg. 1999. 91: 687-90
9. Guinn NR, McDonagh DL, Borel CO, Wright DR, Zomorodi AR, Powers CJ. Adenosine-induced transient asystole for intracranial aneurysm surgery: A retrospective review. J Neurosurg Anesthesiol. 2011. 23: 35-40
10. Heppner PA, Ellegala DB, Robertson N, Nemergut E, Jaganathan J, Mee E. Basilar tip aneurysm-adenosine induced asystole for the treatment of a basilar tip aneurysm following failure of temporary clipping. Acta Neurochir (Wien). 2007. 149: 517-20
11. Intarakhao P, Thiarawat P, Jahromi BR, Kozyrev DA, Teo MK, Choque-Velasquez J. Adenosine-induced cardiac arrest as an alternative to temporary clipping during intracranial aneurysm surgery. J Neurosurg. 2018. 129: 684-90
12. Khan SA, McDonagh DL, Adogwa O, Gokhale S, Toche UN, Verla T. Perioperative cardiac complications and 30-day mortality in patients undergoing intracranial aneurysmal surgery with adenosine-induced flow arrest: A retrospective comparative study. Neurosurgery. 2014. 74: 267-71
13. Lee SH, Kwun BD, Kim JU, Choi JH, Ahn JS, Park W. Adenosine-induced transient asystole during intracranial aneurysm surgery: Indications, dosing, efficacy, and risks. Acta Neurochir (Wien). 2015. 157: 1879-86
14. Luostarinen T, Takala RS, Niemi TT, Katila AJ, Niemela M, Hernesniemi J. Adenosine-induced cardiac arrest during intraoperative cerebral aneurysm rupture. World Neurosurg. 2010. 73: 79-83
15. Meling TR, Romundstad L, Niemi G, Narum J, Eide PK, Sorteberg AG. Adenosine-assisted clipping of intracranial aneurysms. Neurosurg Rev. 2018. 41: 585-92
16. Nimjee SM, McDonagh DL, Agrawal A, Britz GW. A case of high-dose adenosine usage for anterior communicating artery aneurysm clip ligation: What is the dose limit for a resistant response?. Asian J Neurosurg. 2017. 12: 783-6
17. Nussbaum ES, Sebring LA, Ostanny I, Nelson WB. Transient cardiac standstill induced by adenosine in the management of intraoperative aneurysmal rupture: Technical case report. Neurosurgery. 2000. 47: 240-3
18. Owall A, Gordon E, Lagerkranser M, Lindquist C, Rudehill A, Sollevi A. Clinical experience with adenosine for controlled hypotension during cerebral aneurysm surgery. Anesth Analg. 1987. 66: 229-34
19. Owall A, Lagerkranser M, Sollevi A. Effects of adenosine-induced hypotension on myocardial hemodynamics and metabolism during cerebral aneurysm surgery. Anesth Analg. 1988. 67: 228-32
20. Plaschke K, Bockler D, Schumacher H, Martin E, Bardenheuer HJ. Adenosine-induced cardiac arrest and EEG changes in patients with thoracic aorta endovascular repair. Br J Anaesth. 2006. 96: 310-6
21. Powers CJ, Wright DR, McDonagh DL, Borel CO, Zomorodi AR, Britz GW. Transient adenosine-induced asystole during the surgical treatment of anterior circulation cerebral aneurysms: Technical note. Neurosurgery. 2010. 67: 461-70
22. Rankin AC, Oldroyd KG, Chong E, Rae AP, Cobbe SM. Value and limitations of adenosine in the diagnosis and treatment of narrow and broad complex tachycardias. Br Heart J. 1989. 62: 195-203
23. Riksen NP, Smits P, Rongen GA. The cardiovascular effects of methylxanthines. Handb Exp Pharmacol. 2011. 200: 413-37
24. Watt AH, Bernard MS, Webster J, Passani SL, Stephens MR, Routledge PA. Intravenous adenosine in the treatment of supraventricular tachycardia: A dose-ranging study and interaction with dipyridamole. Br J Clin Pharmacol. 1986. 21: 227-30