- Department of Neurosurgery, University of Alberta, Alberta, Canada,
- Department of Radiology, University of Alberta, Alberta, Canada,
- Department of Tyler Research Corporation, Alberta, Canada,
- Department of Anatomy and Cell Biology, University of Alberta, Alberta, Canada,
- Departments of Neurosurgery, King Faisal University, Al-Ahsa, Saudi Arabia,
- Departments of Neurosurgery, King Fahad Medical City, Riyadh, Saudi Arabia.
DOI:10.25259/SNI_532_2020
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Abdullah Alarfaj1,5, Tejas Sankar1, Ravi Bhargava2, Jonathan Tyler3, Anil Walji4, Richard Fox1, Aziz Sagga1,6, Abdullah Ishaque1, Keith Aronyk1. Magnetic resonance imaging analysis of human skull diploic venous anatomy. 31-May-2021;12:249
How to cite this URL: Abdullah Alarfaj1,5, Tejas Sankar1, Ravi Bhargava2, Jonathan Tyler3, Anil Walji4, Richard Fox1, Aziz Sagga1,6, Abdullah Ishaque1, Keith Aronyk1. Magnetic resonance imaging analysis of human skull diploic venous anatomy. 31-May-2021;12:249. Available from: https://surgicalneurologyint.com/surgicalint-articles/10834/
Abstract
Background: The skull diploic venous space (DVS) represents a potential route for cerebrospinal fluid (CSF) diversion and absorption in the treatment of hydrocephalus. The goal of this study was to carry out a detailed characterization of the drainage pattern of the DVS of the skull using high-resolution MRI, especially the diploic veins draining to the lacunae laterales (LLs) since the LLs constitute an important channel for the CSF to access the superior sagittal sinus and subsequently the systemic circulation. The objective was to identify those skull regions optimally suited for an intraosseous CSF diversion system.
Methods: High-resolution, T1-weighted MRI scans from 20 adult and 16 pediatric subjects were selected for analysis. Skulls were divided into four regions, that is, frontal, parietal, temporal, and occipital. On each scan, a trained observer counted all diploic veins in every skull region. Each diploic vein was also followed to determine its final drainage pathway (i.e., dural venous sinus, dural vein, LL, or indeterminate).
Results: In the adult age group, the frontal and occipital skull regions showed the highest number of diploic veins. However, the highest number of draining diploic veins connecting to the lacunae lateralis was found in the frontal and parietal skull region, just anterior and just posterior to the coronal suture. In the pediatric age group, the parietal skull region, just posterior to the coronal suture, showed the highest overall number of diploic veins and also the highest number of draining diploic veins connecting to the LL.
Conclusion: This study suggested that diploic venous density across the skull varies with age, with more parietal diploic veins in the pediatric age range, and more occipital and frontal diploic veins in adults. If the DVS is ultimately used for CSF diversion, our anatomical data point to optimal sites for the insertion of specially designed intraosseous infusion devices for the treatment of hydrocephalus. Likely the optimal sites for CSF diversion would be the parietal region just posterior to the coronal suture in children, and in adults, frontal and/or parietal just anterior or just posterior to the coronal suture.
Keywords: Cerebrospinal fluid diversion, Diploic venous space, Skull regions
INTRODUCTION
Hydrocephalus is one of the oldest and most common problems faced by neurosurgeons.[
Our research group has studied CSF absorption into the venous system through the major cranial venous sinuses for over 20 years.[
The diploic space of the skull is the space between the outer and inner tables of the skull and contains a rich venous vascular network that connects directly to the major dural venous sinuses.[
This current study seeks to build on previous MRI localization work and determines potential intraosseous skull infusion sites for the diversion of CSF in the setting of hydrocephalus. Specifically, we analyzed and characterized the normal in vivo anatomy of the human DVS using MRI scans obtained in both adult and pediatric subjects.
MATERIALS AND METHODS
Ethical approval
The study was approved by the Health Research Ethics Board of the University of Alberta.
Selection of MRI scans for analysis
Preoperative MRI studies from a random series of neurosurgical patients destined for the intraoperative MRI suite between November 2012 and September 2015 were analyzed. A total of 36 MRI studies were selected based on the following criteria:
Inclusion criteria
The following criteria were included in the study:
Intact skull (male or female) Age >2 years MRI study (1.5T or 3T; 1 mm slice thickness and contiguous) with and without contrast to include entire skull.
Exclusion criteria
The following criteria were included in the study:
Any previous surgery involving the skull (including prior craniotomy) Any abnormality involving the cerebral venous sinuses or the skull, as reported on official radiology reports.
Pediatric patients were defined as those <18 years and >2 years at the time of MRI scanning. Adult patients were >18 years.
MRI analysis
All patients underwent diagnostic MR imaging performed with a 1.5 T or 3 T system (Siemens, Erlangen, Germany) and a phased-array head coil. Each study was performed immediately before and after the administration of 0.1 mmol/kg of gadolinium (Gadovist; Berlex Laboratories, Montville, NJ). The pulse sequence used in all analyzed scans – because it allowed for the best resolution of the diploic venous space (DVS) – was a T1-weighted TurboFLASH magnetization prepared rapid gradient echo sequence tfl3dl_ ns (1000/3.93 [repetition time msec/echo time msec], 15 degree flip angle, 256 × 256 matrix, 230 mm field of view, 1.0 mm section thickness, and 0 mm gap).
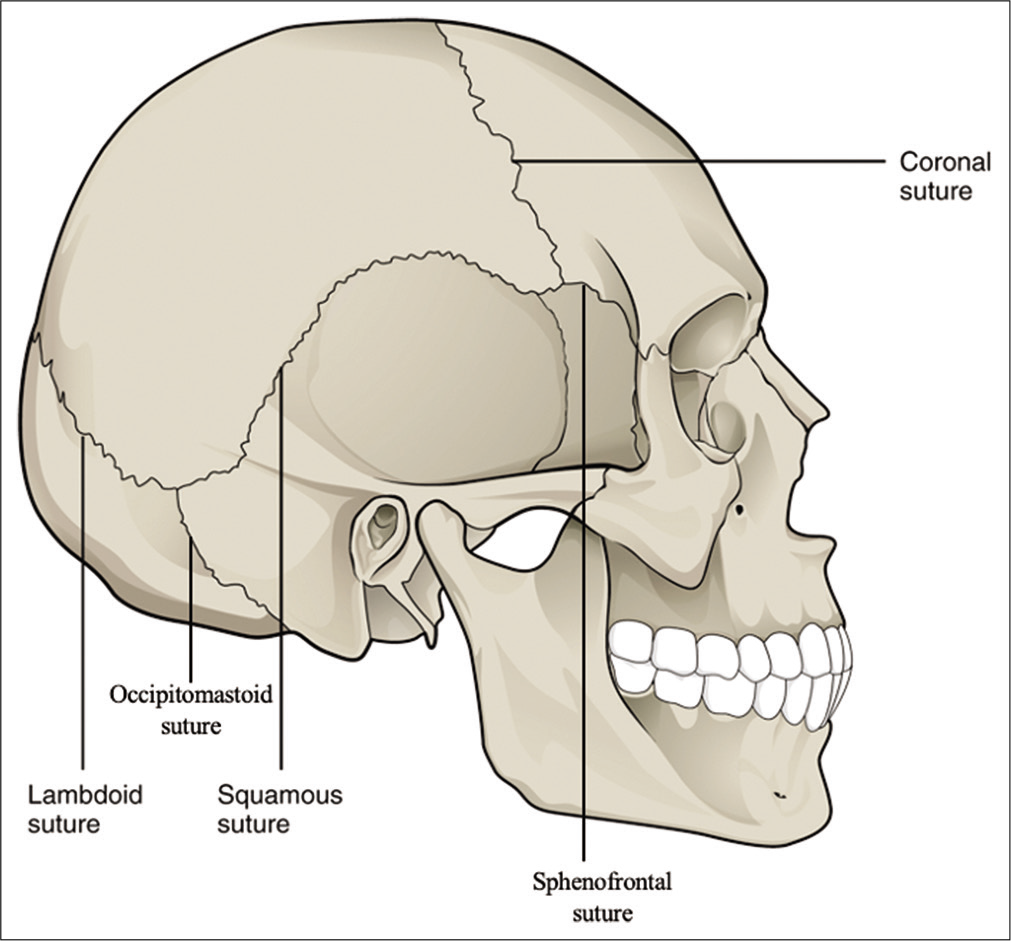
Skulls were divided into four regions corresponding to the underlying lobes, that is, frontal, parietal, temporal, and occipital regions. The frontal skull region was defined as the region of the skull anterior to the coronal suture and superior to the sphenofrontal sutures. The parietal skull region was defined as the region of the skull posterior to the coronal suture and superior to the squamosal suture, as well as anterior/superior to the lambdoid sutures. The temporal skull region was defined as region of the skull inferior to the squamosal and sphenofrontal sutures and anterior to the occipitomastoid suture. The occipital skull region was defined as the region of the skull inferior to the lambdoid suture and posteromedial to the occipitomastoid suture. [
Figure 1:
The definition of skull regions used in the data analysis based on the skull sutures. The frontal skull region was defined as the region of the skull anterior to the coronal suture and superior to the sphenofrontal sutures. The parietal skull region was defined as the region of the skull posterior to the coronal suture and superior to the squamosal suture and anterior/superior to the lambdoid sutures. The temporal skull region was defined as region of the skull inferior to the squamosal and sphenofrontal sutures and anterior to the occipitomastoid suture. The occipital skull region was defined as the region of the skull inferior to the lambdoid suture and posterior to the occipitomastoid suture. Source
All imaging analysis was performed by two observers and the DVS was identified in each skull region, as shown in [
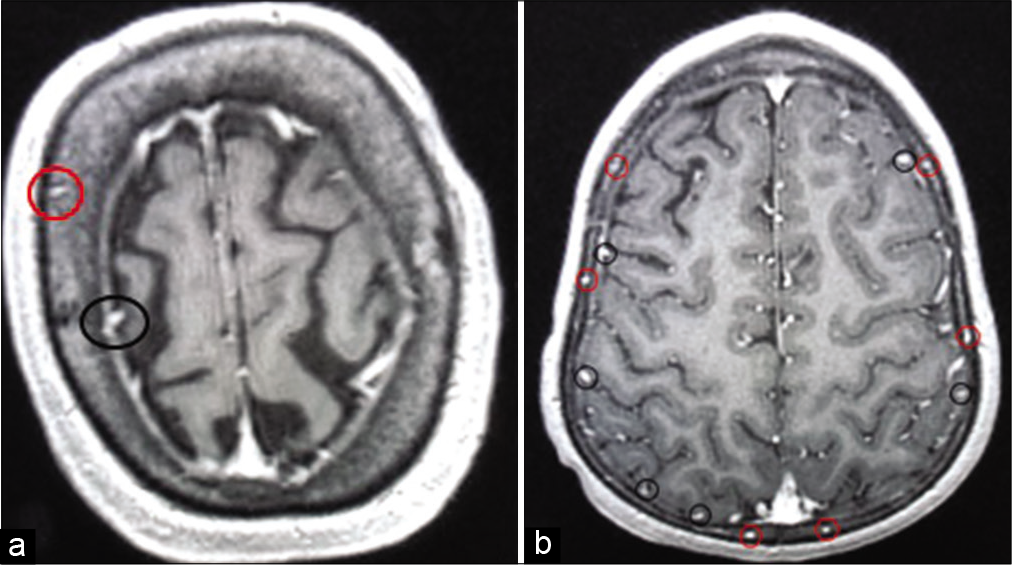
Figure 2:
Illustration of the diploic veins versus cortical veins. The red circles in A and B are identifying the veins coursing in the diploic venous space. Each diploic vein was counted in each skull region and was followed to identify the ultimate pathway. Cortical veins were not counted but are encircled with black circles for illustration.
Drainage pathways were classified as follows:
Draining into a dural venous sinus or dural vein Draining into the LL Indeterminate drainage.
[
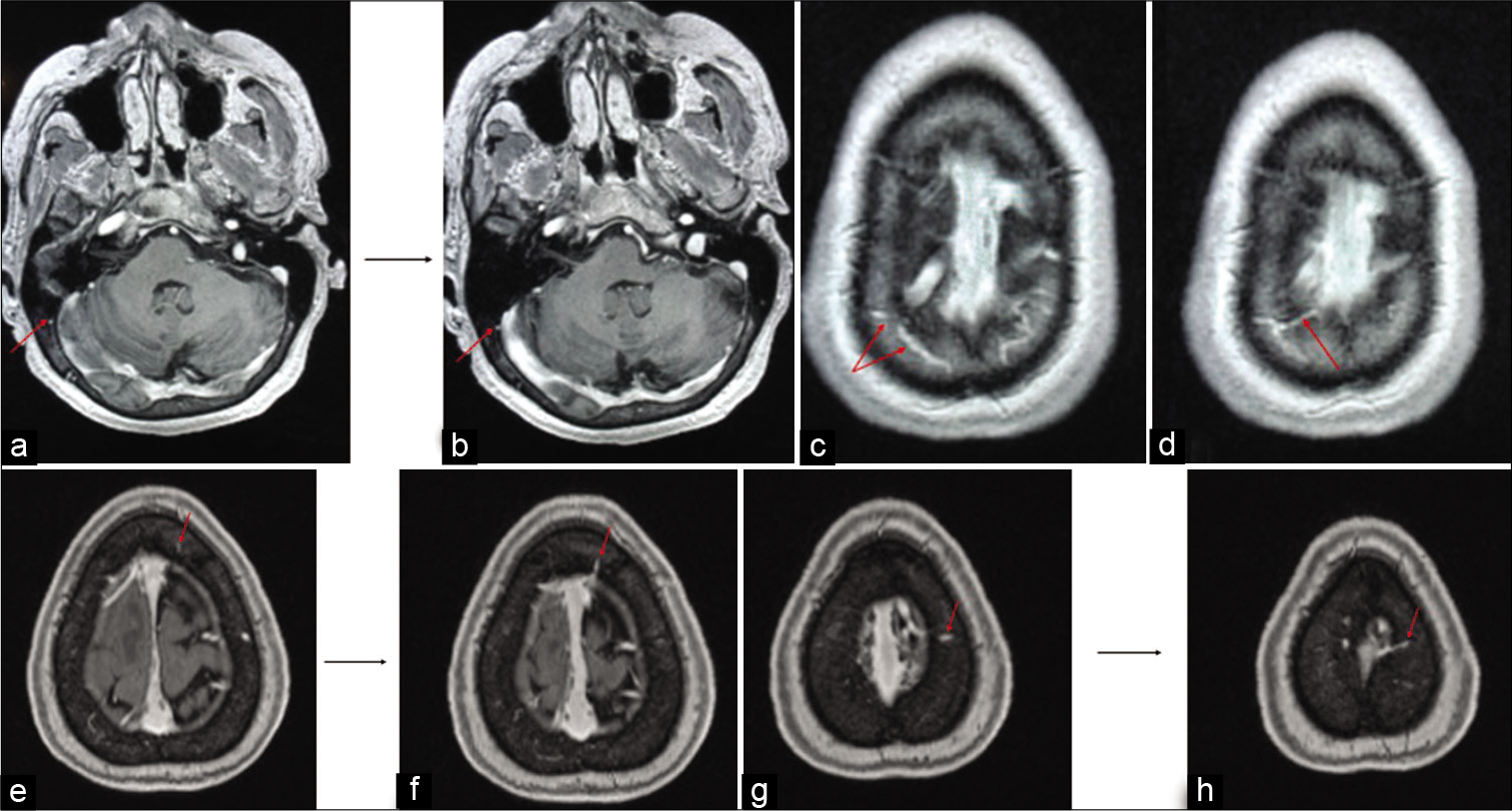
Figure 3:
Illustrations of the different pathways for the diploic veins. The MRI in (a and b) shows a diploic vein in the right occipital region marked with red arrow appearing to drain directly into the transvers sinus. The MRI in (c and d) shows diploic veins marked with red arrow in the right parietal region which appear to drain into the lateral aspect of the superior sagittal sinus. The MRI in (e and f) shows a diploic vein in the left frontal region marked with red arrow appearing to drain into the superior sagittal sinus. The MRI in (g and h) shows a diploic vein in the left parietal region marked with red arrow appearing to drain into the lacunae laterales.
Statistical analysis
There was no difference in mean number of diploic veins between the right and the left right sides for each of the four skull regions (i.e., frontal, parietal, temporal, and occipital, P > 0.05 by paired t-test for all regions), so all subsequent comparisons between skull regions were done using summed right and left vein totals.
The mean number of veins was compared between skull regions using repeated measures analysis of variance with pairwise post hoc comparisons. P < 0.05 was considered statistically significant. Correction for multiple comparisons was carried out using Tukey’s test.
All statistical analyses were carried out using Prism version 8 (GraphPad Software Inc.)
RESULTS
A total of 20 adults brain MRIs and 16 pediatrics brain MRIs were analyzed.
Adult diploic venous anatomy
[
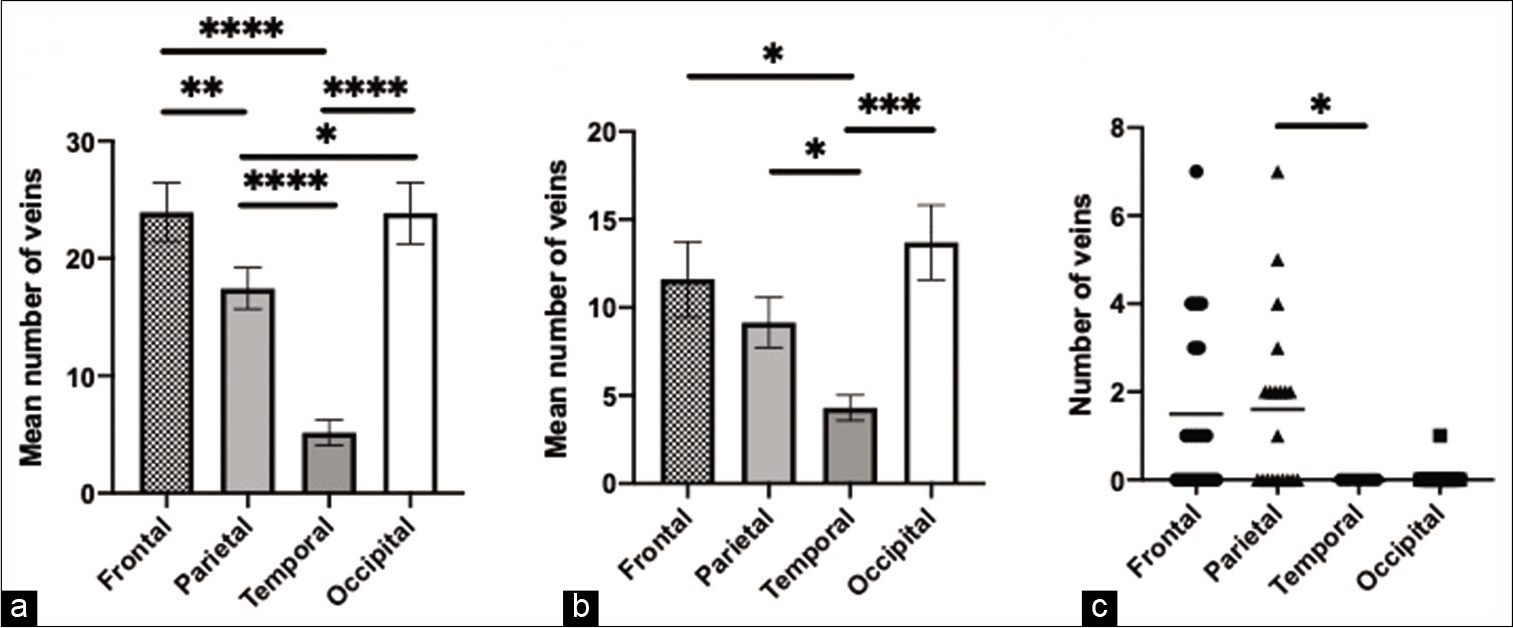
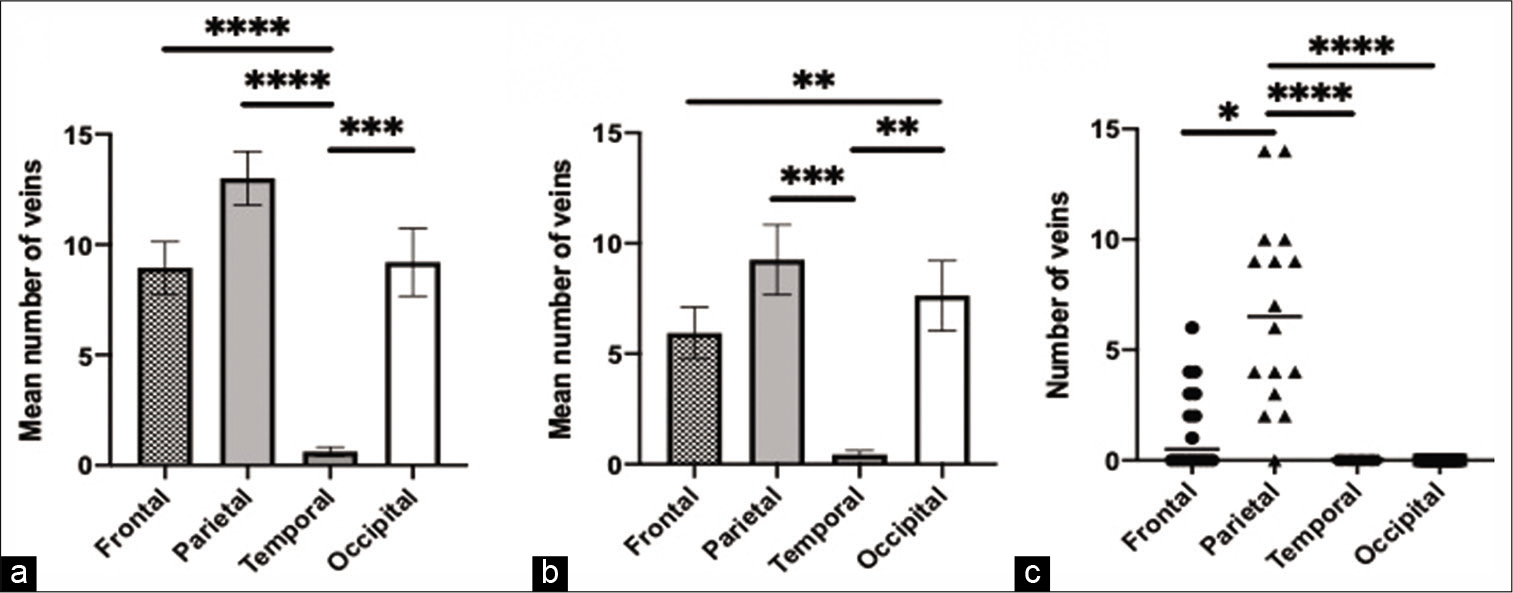
Figure 4:
Adult age group findings. (a) The comparison and significant difference in the mean number of veins in the four skull regions, (b) the comparison and significant difference in the mean number of draining veins in the four skull regions, (c) the comparison and significant difference in the number of veins draining to the lacunae laterales in the four skull regions. * = p<0.05, ** = p<0.01, *** = p<0.001, **** = p<0.0001.
Pediatric diploic venous anatomy
[
Figure 5:
Pediatric age group findings. (a) The comparison and significant difference in the mean number of veins in the four skull regions, (b) the comparison and significant difference in the mean number of draining veins in the four skull regions, (c) the comparison and significant difference in the number of veins draining to the lacunae laterales in the four skull regions. * = p<0.05, ** = p<0.01, *** = p<0.001, **** = p<0.0001.
DISCUSSION
Multiple studies have suggested that the DVS of the skull might serve as a potential absorptive site for CSF to return to the systemic venous system.[
We found that while there was considerable variability in diploic venous anatomy between patients, there were certain patterns that emerged and differed somewhat between adult and pediatric subjects.
When dividing the skull into four regions, across all subjects, we found that the temporal region had the lowest number of diploic veins. In adults, the frontal and occipital regions contained the highest number of diploic veins, including draining diploic veins. Interestingly, in pediatric subjects, the parietal region had more diploic veins including draining veins. Overall, these findings suggest that the DVS in the skull undergoes developmental changes with aging. Further investigation is required to elucidate the developmental trajectory of changes in diploic vein concentration. We speculate that, in adults, the occipital region may exhibit a large number of diploic veins because the occipital bone is the thickest region of the skull.[
Another novel analysis we carried out is a detailed evaluation of diploic veins which drain to the LL. The LLs constitute an important channel for the CSF to access the SSS and subsequently the systemic circulation.[
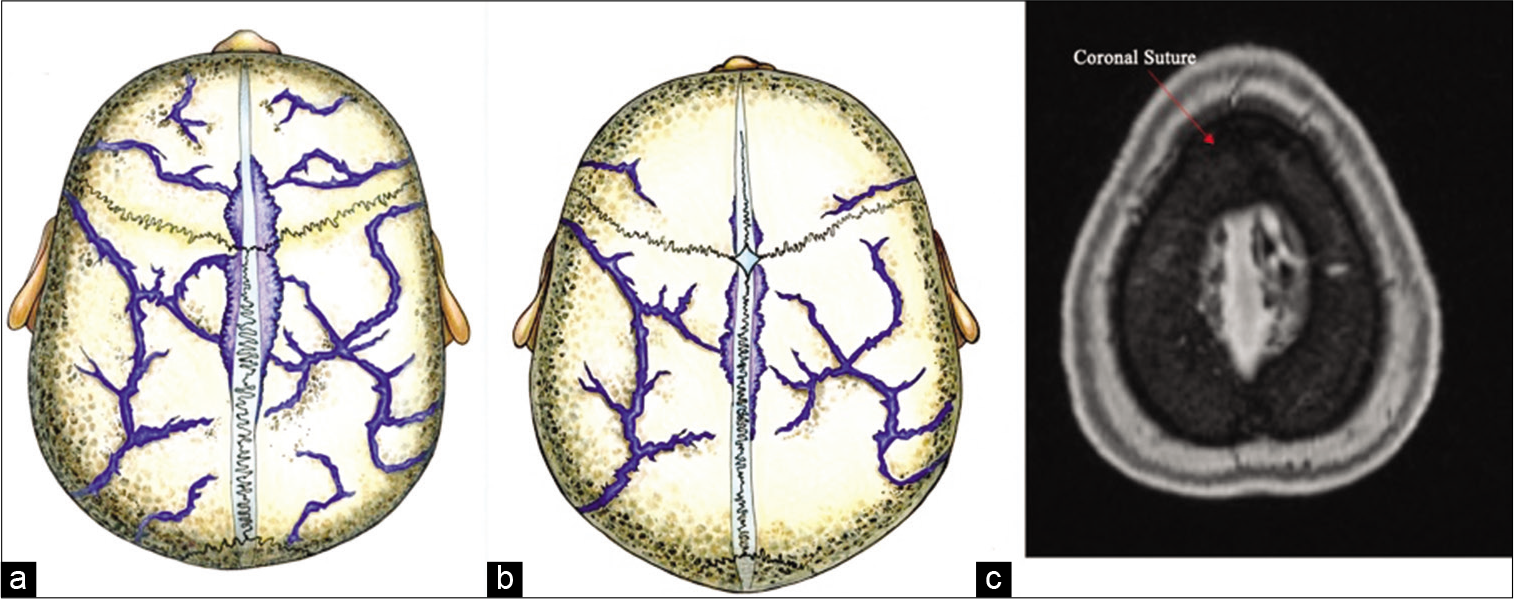
Figure 6:
The Lacuane Laterales in relation to the coronal suture and skull diploic veins. The illustrative pictures in A and B shows the anatomic location of the LL just adjacent to the coronal suture which corresponds with the radiological finding of the highest number of diploic veins draining to the LL just anterior and just posterior to the coronal suture, in the adult age group and the pediatric age group, respectively. Please refer to Rhoton Cranial Anatomy and Surgical Approaches text book figure 4.14 a page 179 and figure 4.3 c page 164 for cadaveric dissection showing LL in relation to coronal suture. (a) Illustration showing the LL in relation to the coronal suture in adults. (b) Illustration showing the LL in relation to the coronal suture in pediatrics. (c) MRI MP RAGE showing the LL in relation to the coronal suture “red arrow pointing at the coronal suture.”
It is worth mentioning that multiple recent studies have suggested that the DVS contributes to CSF drainage.[
Bulleid et al. published an important case report regarding the absorption capacity of CSF by the DVS.[
Ultimately, while our anatomical study of diploic venous anatomy contributes important information in the quest to develop an alternate route for CSF absorption, our study was limited by a small and somewhat selected sample. Our methodology could be improved by fully randomizing our patient selection and including volunteers from the general population.
CONCLUSION
Previous work in our laboratory has suggested that the DVS might provide a route for CSF diversion. The current anatomical MRI study suggests some potential sites for the insertion of specially designed intraosseous skull infusion devices which can take advantage of the DVS, though much work remains before such devices become clinically viable.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to thank the iMRI technician Kim Perry and Angela Edwards RN for their contributions.
References
1. Aschoff A, Kremer P, Hashemi B, Kunze S. The scientific history of hydrocephalus and its treatment. Neurosurg Rev. 1999. 22: 67-93
2. Baert EJ, Dewaele F, Vandersteene J, Hallaert G, Kalala JO, van Roost D. Treating hydrocephalus with retrograde ventriculosinus shunt: Prospective clinical study. World Neurosurg. 2018. 118: e34-42
3. Brinker T, Stopa E, Morrison J, Klinge P. A new look at cerebrospinal fluid circulation. Fluids Barriers CNS. 2014. 11: 10
4. Bulleid LS, Hughes T, Bhatti I, Leach PA. Low-pressure headaches following foramen magnum decompression secondary to absorption of cerebrospinal fluid into the venous system of the diploic space. Childs Nerv Syst. 2016. 32: 897-9
5. Fox RJ, Walji AH, Mielke B, Petruk KC, Aronyk KE. Anatomic details of intradural channels in the parasagittal dura: A possible pathway for flow of cerebrospinal fluid. Neurosurgery. 1996. 39: 84-90
6. Hanak BW, Bonow RH, Harris CA, Browd SR. Cerebrospinal fluid shunting complications in children. Pediatr Neurosurg. 2017. 52: 381-400
7. Jivraj K, Bhargava R, Aronyk K, Quateen A, Walji A. Diploic venous anatomy studied in vivo by MRI. Clin Anat. 2009. 22: 296-301
8. Johnston KD, Walji AH, Fox RJ, Pugh JA, Aronyk KE. Access to cerebrospinal fluid absorption sites by infusion into vascular channels of the skull diplo. J Neurosurg. 2007. 107: 841-3
9. Li Z, Park BK, Liu W, Zhang J, Reed MP, Rupp JD. A statistical skull geometry model for children 0-3 years old. PLoS One. 2015. 10: e0127322
10. Mahinda H, Murty OP. Variability in thickness of human skull bones and sternum-an autopsy experience. J Forensic Med Toxicol. 2009. 26: 26-31
11. Paff M, Alexandru-Abrams D, Muhonen M, Loudon W. Ventriculoperitoneal shunt complications: A review. Int Neurosurg. 2018. 13: 66-70
12. Prakash P, Dhandapani M, Ghai S, Singh NV, Dhandapani S. Quality of life among children who had undergone ventriculoperitoneal shunt surgery. J Pediatr Neurosci. 2018. 13: 189-94
13. Pugh JA, Tyler J, Churchill TA, Fox RJ, Aronyk KE. Intraosseous infusion into the skull: Potential application for the management of hydrocephalus. J Neurosurg. 2007. 106: 120-5
14. Robertson JS, Maraqa MI, Jennett B. Ventriculoperitoneal shunting for hydrocephalus. Br Med J. 1973. 2: 289-92
15. Toriumi H, Shimizu T, Shibata M, Unekawa M, Tomita Y, Tomita M. Developmental and circulatory profile of the diploic veins. Microvasc Res. 2011. 81: 97-102
16. Tsutsumi S, Nakamura M, Tabuchi T, Yasumoto Y, Ito M. Calvarial diploic venous channels: An anatomic study using high-resolution magnetic resonance imaging. Surg Radiol Anat. 2013. 35: 935-41
17. Tsutsumi S, Ogino I, Miyajima M, Ito M, Arai H, Yasumoto Y. Cerebrospinal fluid drainage through the diploic and spinal epidural veins. J Anat. 2015. 227: 297-301
18. Tsutsumi S, Ogino I, Miyajima M, Nakamura M, Yasumoto Y, Arai H. Cranial arachnoid protrusions and contiguous diploic veins in CSF drainage. AJNR Am J Neuroradiol. 2014. 35: 1735-9
19. Tsutsumi S, Ono H, Ishii H, Yasumoto Y. Diploic veins of the cranial base: An anatomical study using magnetic resonance imaging. Surg Radiol Anat. 2019. 41: 1029-36