- Department of Radiodiagnosis, Katuri Medical College, Guntur, Andhra Pradesh, India.
Correspondence Address:
Ramakrishna Narra, Department of Radiodiagnosis, Katuri Medical College, Guntur, Andhra Pradesh, India.
DOI:10.25259/SNI_1081_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ramakrishna Narra, Suseel Kumar Kamaraju. Magnetic resonance imaging features of “Proximal” hirayama disease: Case report and literature review. 20-Dec-2021;12:622
How to cite this URL: Ramakrishna Narra, Suseel Kumar Kamaraju. Magnetic resonance imaging features of “Proximal” hirayama disease: Case report and literature review. 20-Dec-2021;12:622. Available from: https://surgicalneurologyint.com/surgicalint-articles/11296/
Abstract
Background: Proximal “Hirayama” disease (PHD) is characterized by proximal upper extremity atrophy. It is a rare variant of Hirayama disease (HD) which involves the proximal upper limb. Recognition of PHD’s unique magnetic resonance (MR) findings is critical as the treatment options differ versus classical HD.
Case Description: A 17-year-old male presented with gradual progressive upper extremity weakness and atrophy. On MR, PHD was demonstrated by C4-C5 kyphosis with a posterior epidural soft-tissue mass compressing the C4-C5 cord resulting in gliosis. As the patient declined surgery, he was followed for 1 year with a cervical collar during which time his deficit stabilized.
Conclusion: PHD, characterized by proximal upper extremity weakness and atrophy, has characteristic MR findings of kyphosis associated with cord compression and ischemia/gliosis. Select patients as the one we described who decline surgery may stabilize radiographically and clinically with the protracted utilization of a cervical collar.
Keywords: Epidural soft tissue, Flexion magnetic resonance imaging, Hirayama disease, Magnetic resonance imaging, Proximal type Hirayama disease
INTRODUCTION
Proximal “Hirayama” disease (PHD) is characterized by proximal upper extremity atrophy. It is unlike classical Hirayama disease (HD) which involves the distal acral part of the upper limb. Magnetic resonance (MR) findings include posterior cervical epidural soft-tissue lesions resulting in cord compression/gliosis along with kyphosis.[
CASE PRESENTATION
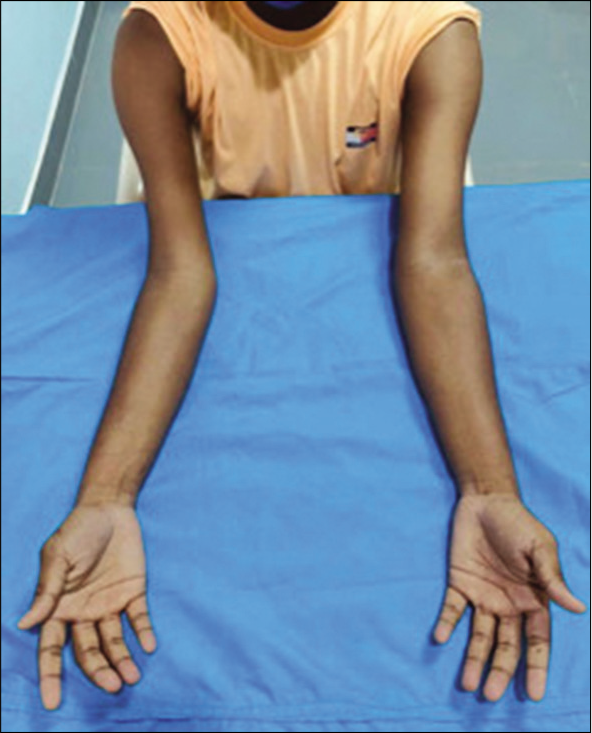
A 17-year-old adolescent male presented with 3 months of gradually progressive right upper limb weakness (3/5) and atrophy (i.e., involving the muscles of arm and forearm muscles) [
Electromyelography (EMG)
The needle EMG of the muscles of the right arm and forearm showed polyphasic motor unit action potentials with increased amplitudes and delayed latencies.
MR
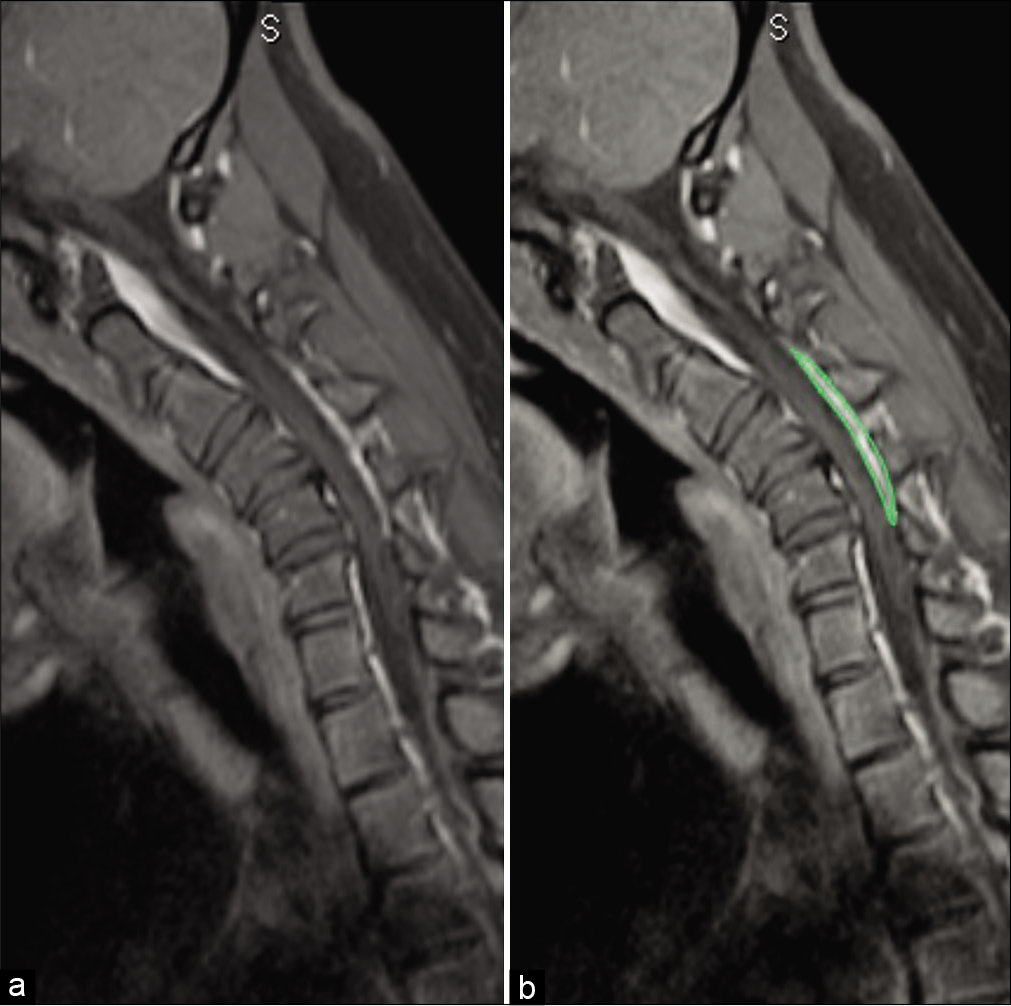
The cervical MR showed cord compression, flattening, and atrophy with kyphosis from C2 to C6 maximum at C4-C5 apex [
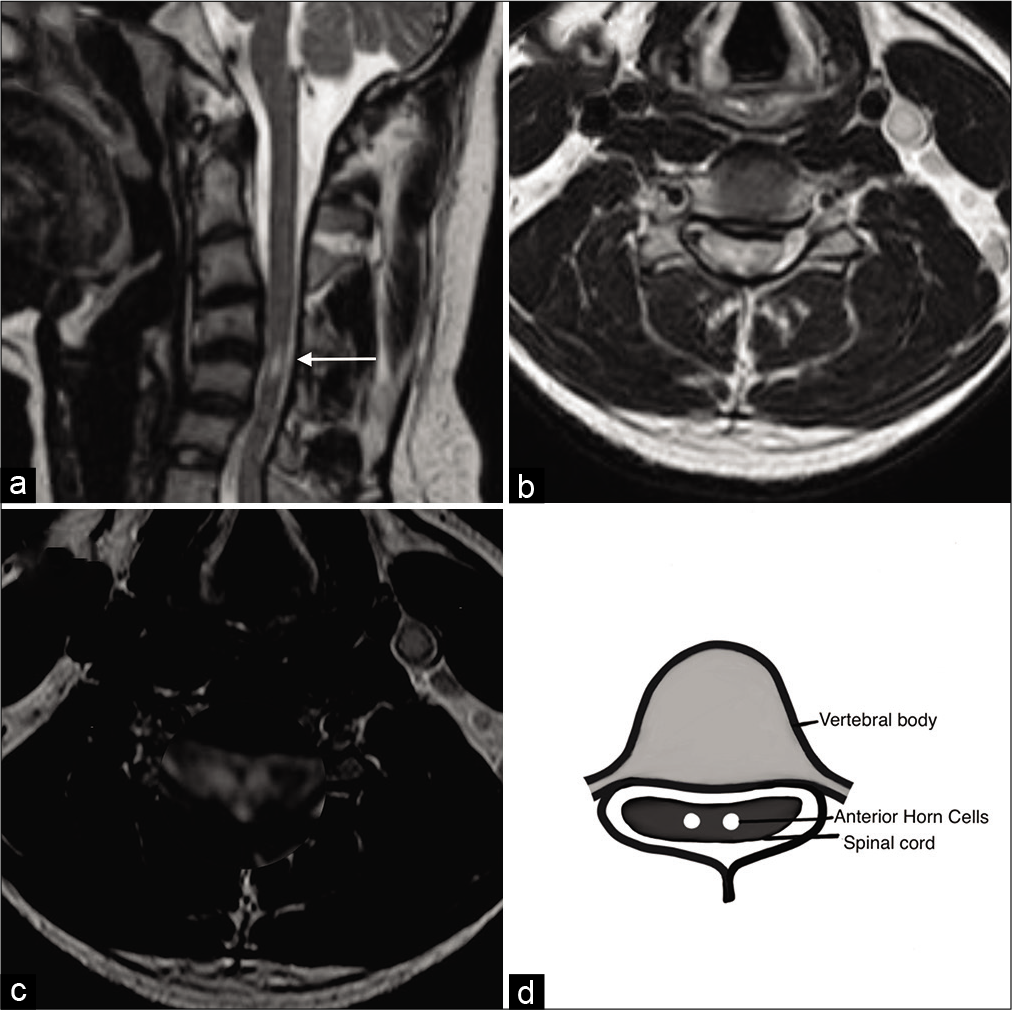
Figure 2:
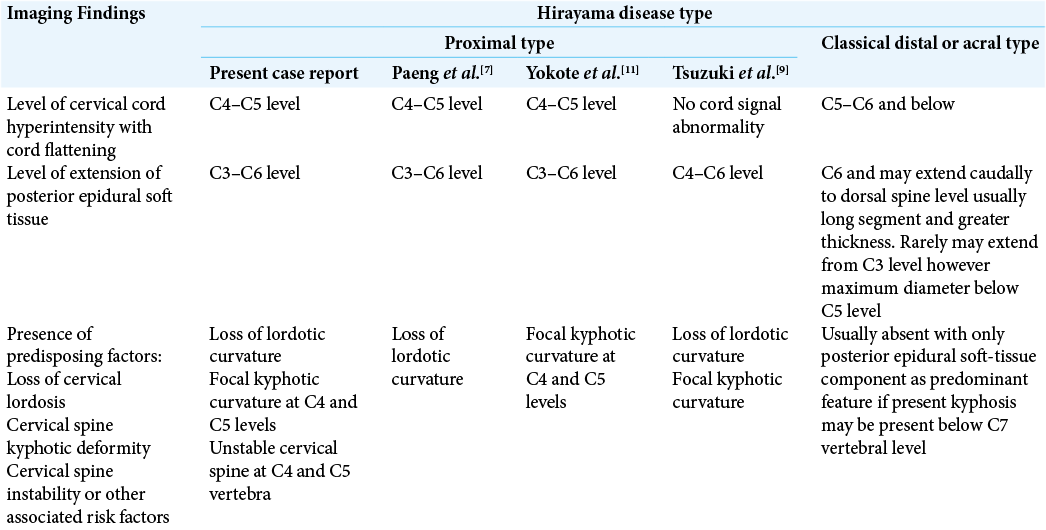
(a) Sagittal T2W magnetic resonance imaging (MRI) in neutral position image demonstrating loss of normal lordotic curvature with focal kyphotic angulation of the cervical spine with increased signal intensity in the cervical spinal cord at C4-C5 intervertebral level. Degeneration of intervertebral disks noted at multiple levels. (b) Axial MRI T2W image demonstrating typical snake eye hyperintense signals of the cervical spinal cord at C4-C5 level suggestive of subacute spinal cord ischemia. (c) Enlarged (zoomed in) Axial MRI T2W image demonstrating typical “snake eye” hyperintensity in the anterior horn cells of the spinal cord, (d) Diagrammatic representation of atrophied and gliosed anterior horn cells demonstrating snake eye appearance.
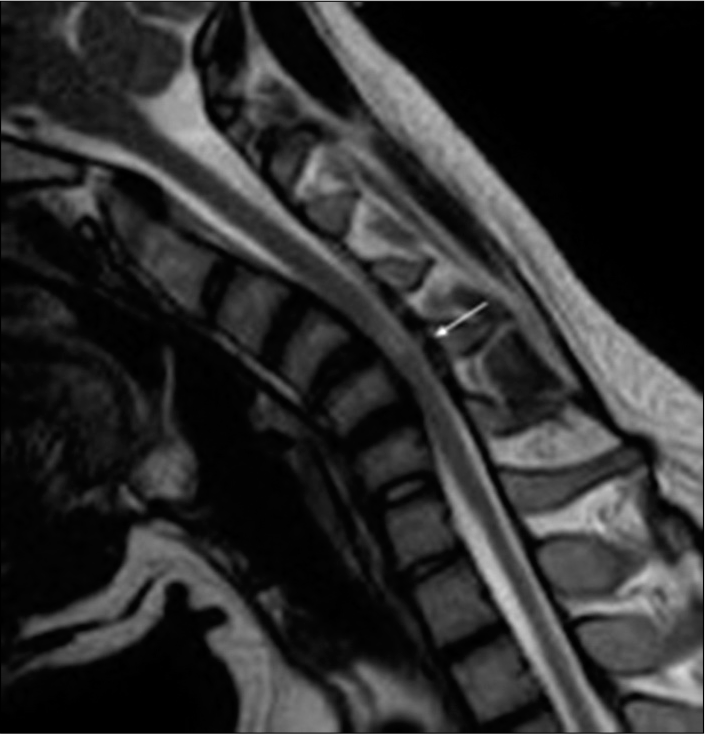
Figure 3:
Sagittal T2W magnetic resonance imaging in flexion position demonstrating prominent posterior epidural soft-tissue component (arrow) displacing the posterior dural sac anteriorly and causing compression of the spinal cord with increased signal intensity(myelomalacia/subacute infarct) at C4 and C5 vertebral levels. Note: the apex of compression at C4 and C5 levels in the spinal canal causing maximum cord compression due to the kyphotic curvature.
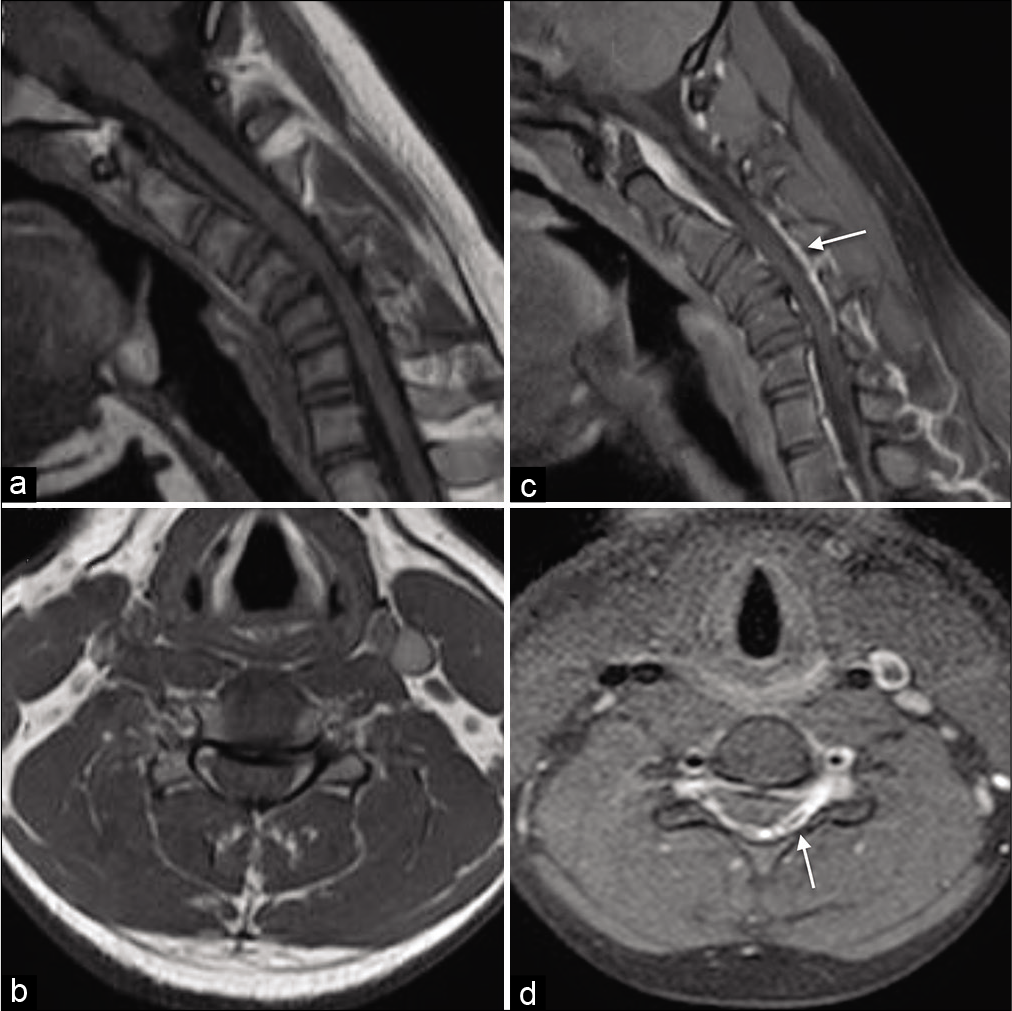
Figure 4:
Pre contrast T1W magnetic resonance (MR) sagittal (a) and axial image (b) in flexion position demonstrating prominent posterior epidural space displacing the posterior dural sac anteriorly and causing compression of the spinal cord. Post contrast T1W MR sagittal (c) and axial image (d) in flexion demonstrating homogeneous enhancement in the posterior epidural space at C3–C6 vertebral levels (Arrows).
Diagnosis of PHD
Based on the clinical presentation and dynamic MRI findings, a diagnosis of PHD was established. The patient refused to undergo surgery. Therefore, he was managed with a cervical collar for 1 year during which time his deficit and MR findings stabilized.
DISCUSSION
HD is a form of cervical myelopathy that commonly occurs in young adolescents predominantly males (M: F = 3:1).[
MR findings of PHD
MRI is the preferred technique for diagnosis of HD.[
Treatment
The treatment for PHD is cervical decompression with fusion to prevent progression.[
CONCLUSION
PHD a rare variant of HD is characterized by proximal upper extremity atrophy. Its unique clinical and MR findings help differentiate it from classical HD.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Hirayama K, Tokumaru Y. Cervical dural sac and spinal cord in juvenile muscular atrophy of distal upper extremity. Neurology. 2000. 54: 1922-26
2. Hirayama K. Juvenile muscular atrophy of unilateral upper extremity (Hirayama disease) Half-century progress and establishment since its discovery. Brain Nerve. 2008. 60: 17-29
3. Imamura H, Matsumoto S, Hayase M, Oda Y, Kikuchi H, Takano M. A case of Hirayama’s disease successfully treated by anterior cervical decompression and fusion. No To Shinkei. 2001. 53: 1033-8
4. Kim J, Kim Y, Kim S, Oh K. Hirayama disease with proximal involvement. J Korean Med Sci. 2016. 31: 1664-7
5. Lai V, Wong YC, Poon WL, Yuen MK, Fu YP, Wong OW. Forward shifting of posterior dural sac during flexion cervical magnetic resonance imaging in Hirayama disease: An initial study on normal subjects compared to patients with Hirayama disease. Eur J Radiol. 2011. 80: 724-8
6. Lehman VT, Luetmer PH, Sorenson EJ, Carter RE, Gupta V, Fletcher GP. Cervical spine MR imaging findings of patients with Hirayama disease in North America: A multisite study. Am J Neuroradiol. 2013. 34: 451-6
7. Paeng SH, kim YJ, Oh SI, Bae JS. Predominant proximal upper extremity involvement in Hirayama disease. Neurol Asia. 2015. 20: 301-3
8. Prior DE, Ghosh PS. Neuroimage: The crescent moon sign of Hirayama disease. Acta Neurol Belg. 2021. p.
9. Tsuzuki U, Ando T, Sugiura M, Kawakami O. A case of proximal-type Hirayama disease associated with neck axial rotation. Rinsho Shinkeigaku. 2021. 61: 120-6
10. Xu H, Shao M, Zhang F, Nie C, Wang H, Zhu W. Snake-eyes appearance on MRI occurs during the late stage of Hirayama disease and indicates poor prognosis. Biomed Res Int. 2019. 2019: 9830243
11. Yokote A, Fukuhara K, Tsugawa J, Tsuboi Y. Juvenile muscular atrophy of the proximal upper extremity as so-called proximal-type Hirayama disease: Case report and review of the literature. Case Rep Neurol. 2019. 11: 106-11