- Department of Neurological Surgery, The Ohio State University Wexner Medical Center, Columbus, OH 43210, USA
- Department of Otolaryngology - Head and Neck Surgery, The Ohio State University Wexner Medical Center, Columbus, OH 43210, USA
- Department of Neurological Surgery, University of Pittsburgh Medical Center, UPMC Presbyterian, Pittsburgh, PA 15213, USA
- Department of Neurological Sciences, Division of Neurosurgery, University of Napoli Federico II, 80131 Naples, Italy
- Department of Neurological Surgery, University of São Paulo, Central Institute of the University of São Paulo Medical School Clinical Hospital, São Paulo, Brazil
- Department of Biomedical Informatics, Center for Biostatistics, College of Medicine, The Ohio State University, Columbus, OH 43221, USA
- Department of Neurosurgery, Aurora Neuroscience Innovation Institute, Milwaukee, WI 53215, USA
Correspondence Address:
Daniel M. Prevedello
Department of Neurosurgery, Aurora Neuroscience Innovation Institute, Milwaukee, WI 53215, USA
DOI:10.4103/2152-7806.166846
Copyright: © 2015 Prevedello DM. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.How to cite this article: Prevedello DM, Ditzel Filho LF S, Fernandez-Miranda JC, Solari D, Marcelo Prudente do Espírito Santo, Wehr AM, Carrau RL, Kassam AB. Magnetic resonance imaging fluid-attenuated inversion recovery sequence signal reduction after endoscopic endonasal transcribiform total resection of olfactory groove meningiomas. Surg Neurol Int 07-Oct-2015;6:158
How to cite this URL: Prevedello DM, Ditzel Filho LF S, Fernandez-Miranda JC, Solari D, Marcelo Prudente do Espírito Santo, Wehr AM, Carrau RL, Kassam AB. Magnetic resonance imaging fluid-attenuated inversion recovery sequence signal reduction after endoscopic endonasal transcribiform total resection of olfactory groove meningiomas. Surg Neurol Int 07-Oct-2015;6:158. Available from: http://surgicalneurologyint.com/surgicalint_articles/magnetic-resonance-imaging-fluid%e2%80%91attenuated-inversion/
Abstract
Background:Olfactory groove meningiomas grow insidiously and compress adjacent cerebral structures. Achieving complete removal without further damage to frontal lobes can be difficult. Microsurgical removal of large lesions is a challenging procedure and usually involves some brain retraction. The endoscopic endonasal approaches (EEAs) for tumors arising from the anterior fossa have been well described; however, their effect on the adjacent brain tissue has not. Herein, the authors utilized the magnetic resonance imaging fluid attenuated inversion recovery (FLAIR) sequence signal as a marker for edema and gliosis on pre- and post-operative images of olfactory groove meningiomas, thus presenting an objective parameter for brain injury after surgical manipulation.
Methods:Imaging of 18 olfactory groove meningiomas removed through EEAs was reviewed. Tumor and pre/postoperative FLAIR signal volumes were assessed utilizing the DICOM image viewer OsiriX®. Inclusion criteria were: (1) No previous treatment; (2) EEA gross total removal; (3) no further treatment.
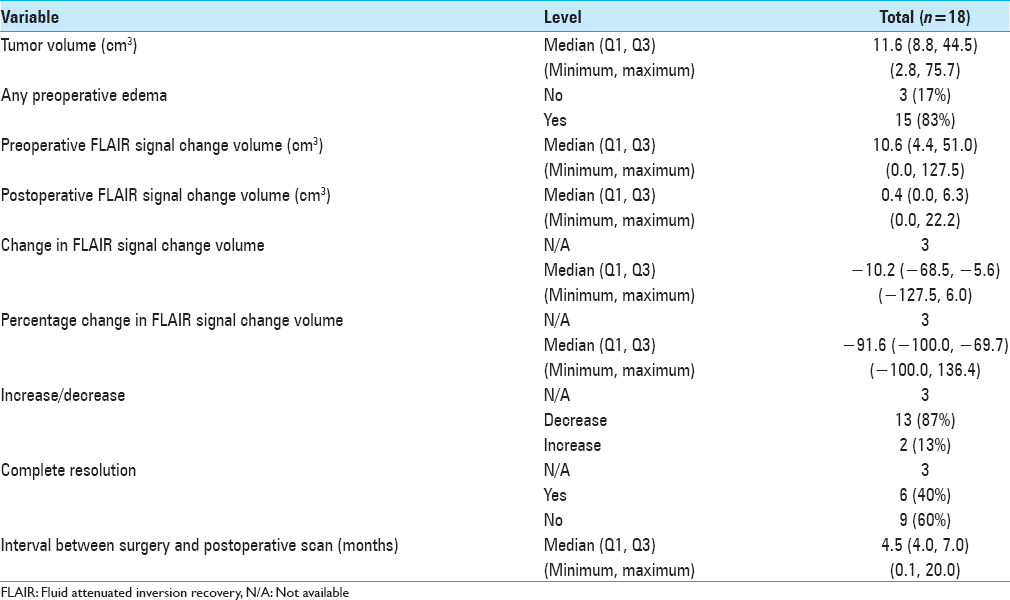
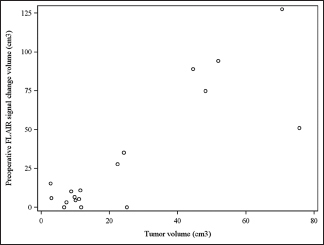
Results:There were 14 females and 4 males; the average age was 53.8 years (±8.85 years). Average tumor volume was 24.75 cm3 (±23.26 cm3, range 2.8-75.7 cm3), average preoperative FLAIR volume 31.17 cm3 (±39.38 cm3, range 0-127.5 cm3) and average postoperative change volume, 4.16 cm3 (±6.18 cm3, range 0-22.2 cm3). Average time of postoperative scanning was 6 months (range 0.14-20 months). In all cases (100%) gross total tumor removal was achieved. Nine patients (50%) had no postoperative FLAIR changes. In 2 patients (9%) there was minimal increase of changes postoperatively (2.2 cm3 and 6 cm3 respectively); all others demonstrated image improvement. The most common complication was postoperative cerebrospinal fluid leakage (27.8%); 1 patient (5.5%) died due to systemic complications and pulmonary sepsis.
Conclusions:FLAIR signal changes tend to resolve after endonasal tumor resection and do not seem to worsen with this operative technique.
Keywords: Anterior cranial fossa, endonasal endoscopic, fluid-attenuated inversion recovery, magnetic resonance imaging, meningioma, olfactory groove
INTRODUCTION
Olfactory groove meningiomas can grow insidiously and significantly compress the adjacent cerebral structures. One of the main challenges in their surgical management is achieving complete removal without further damage to the frontal lobes. Microsurgical removal of large olfactory groove meningiomas is still a challenging procedure even for the most skilled neurosurgeon, since it usually involves a significant amount of brain retraction that may result in damage to normal tissue surrounding the tumor. This damage due to manipulation of edematous, frail and yet viable brain tissue may lead to postoperative maintenance of subtle neurological impairments or even create new ones.
Several approaches have been proposed for the transcranial resection of these tumors, with varying results;[
The authors utilized the analysis of magnetic resonance imaging (MRI) fluid-attenuated inversion recovery (FLAIR) sequence signal as a marker for edema and gliosis on pre- and postoperative images of olfactory groove meningiomas, thus presenting an objective parameter for brain damage after surgical removal of these tumors. Through this method, they attempt to determine the cerebral impact of endonasal resection of these lesions.
PATIENTS AND METHODS
Patient cohort selection
Patients with olfactory groove meningiomas were selected from a database of over 1000 patients who underwent endonasal resection at the University of Pittsburgh Medical Center between July 1998 and April 2008. The inclusion criteria were:
Patients with olfactory groove meningioma with no previous treatment Endonasal gross total removal with MRI confirmation Absence of further treatment.
The authors opted to include only cases of gross total resection to avoid interference of residual tumor on the maintenance or propagation of postoperative FLAIR changes. Medical records and imaging studies provided data regarding the pathological entity, surgical technique, tumor volumes in preoperative images as well as FLAIR volumes in pre- and post-operative images; only patients in which pathology confirmed the diagnosis of meningioma and were submitted to a fully endoscopic, endonasal resection of their tumor per the following technique were included in this study.
Surgical technique
The surgical technique and nuances of the endonasal transcribiform approach are not new; they have been described in detail by the authors and others.[
After endotracheal intubation the patient is placed supine and the head is secured with a three-point Mayfield headholder. The neck is extended; the head is turned to the right and tilted to the left. It is imperative to properly position to patient in order to gain access to the most anterior aspect of the skull base. Somatosensory evoked potentials monitoring is performed in all patients.
Nasal preparation begins by packing oxymetazoline 0.025% - soaked pledgets into both nasal cavities. The external portion of the nose is cleaned with povidone solution and a fourth generation cephalosporin antibiotic is administered for perioperative prophylaxis, during anesthetic induction.
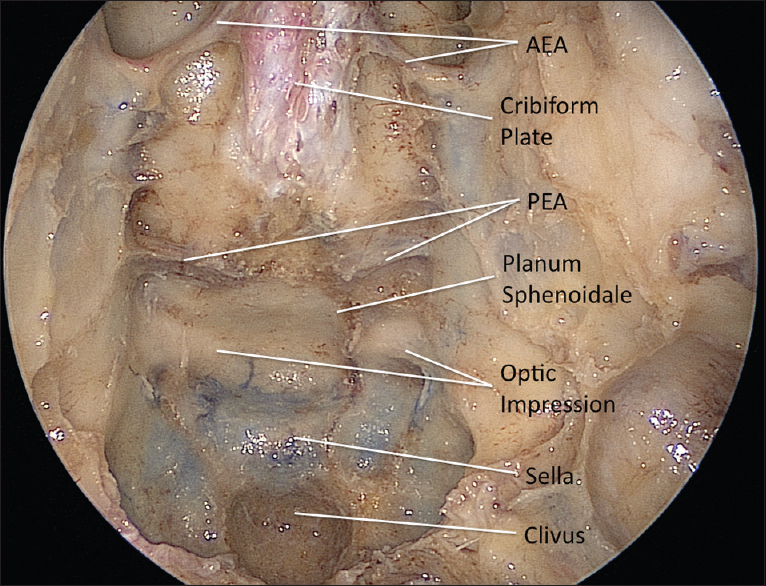
The initial exposure involves expanding the nasal corridor; a 0° endoscope is utilized in the first stages of the approach. The procedure is initiated with the removal of the right middle turbinate in order to amplify the surgical corridor and enhance instrument maneuverability. A portion of the nasal septum is removed posteriorly and wide bilateral sphenoidotomies are performed, extending laterally and inferiorly to the level of the medial pterigoid plates. 1–2 cm of the posterior nasal septum is resected, thus creating a single operative cavity. A wide sphenoidectomy will facilitate the identification and exposure of surgical landmarks including the planum sphenoidale, clival recess, internal carotid arteries, optic nerve canals and the medial and lateral optical-carotid recesses [
After this basic exposure, the dissection proceeds to the transcribiform approach itself. This approach extends the previous exposure rostrally to the level of the crista galli. It may be combined with an endoscopic Draf III procedure (frontal sinusotomy) to access the back wall of the frontal sinus. The posterior nasal septum attachment to the ventral skull base is resected, and the anterior and posterior ethmoidal arteries are identified and coagulated to provide tumor devascularization. The fronto-ethmoidal recess is identified, and the skull base is drilled in a rostral-caudal direction. Removal of the crista galli and cribiform plate in addition to the transplanum approach creates a single cavity along the anterior skull base. The anterior margin of the exposure is the frontal sinus; the posterior boundary is formed by the planum sphenoidale and the lateral margins by the lamina papyracea on both sides. Following coagulation, the dura mater is opened separately on both sides of the falx. The midline portion is left intact as it has vascular supply from the anterior falcine artery. The free edge of the falx is identified from both sides and coagulated before transecting it to create a single intradural cavity.
The tumor is then incised in its middle section and its internal contents resected with a combination of two suctions, sharp dissection and mechanical removal by a side-cutting aspiration device.[
This technique enabled the authors to achieve gross total resection of all the tumors in the present cohort [
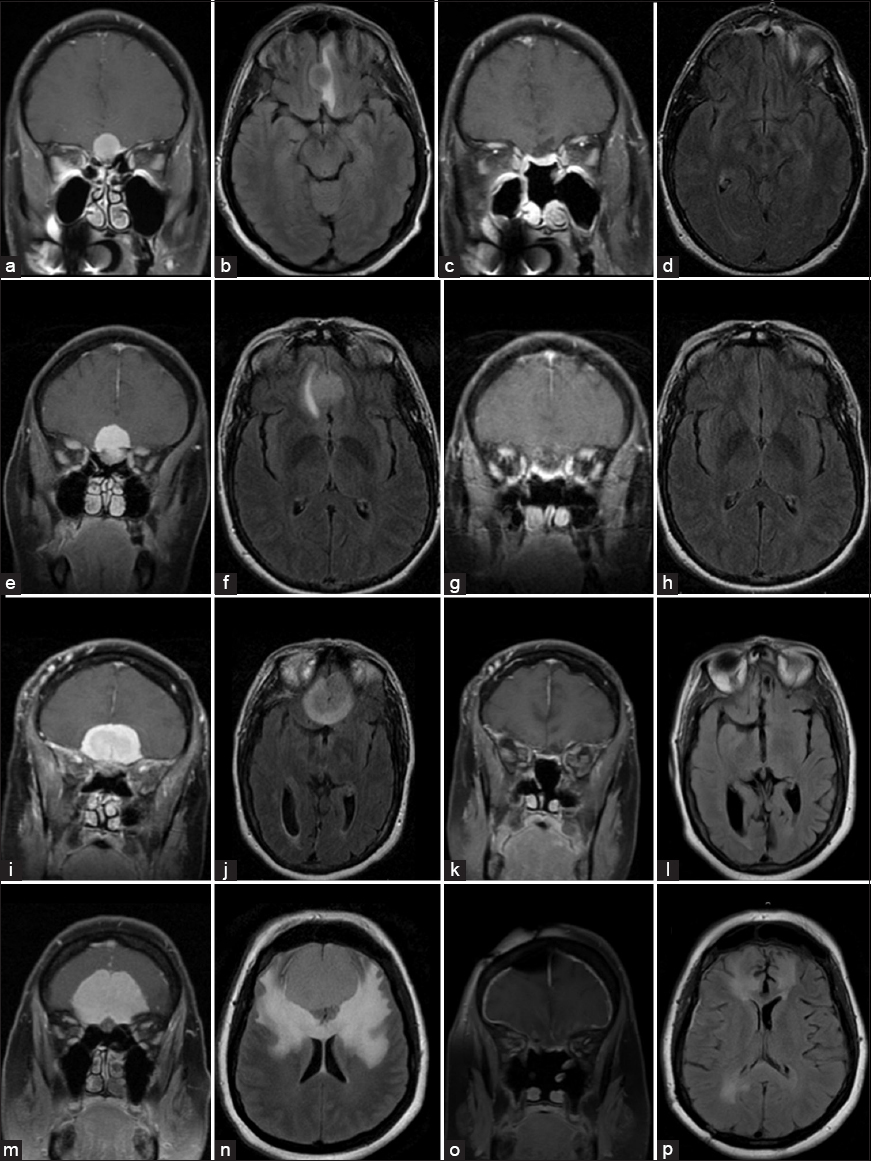
Figure 2
Pre- (left 2 columns) and post-operative (right 2 columns) magnetic resonance T1 contrast coronal (a, c, e, g, i, k, m, o) and T2 axial fluid attenuated inversion recovery (b, d, f, h, j, l, n, p) imaging of four examples of olfactory groove meningiomas completely resected through an endoscopic endonasal approaches. Control imaging was performed on average at 6 months after surgery and confirmed either absence of new signal changes or near total resolution of preoperative signal changes
Data collection and statistical analysis
Pre- and post-operative MRI studies of 18 olfactory groove meningiomas completely removed through EEAs were reviewed. Tumor volume as well as pre- and post-operative FLAIR signal change volumes were calculated utilizing the DICOM image viewer OsiriX® (Pixmeo, Geneva, Switzerland) volume rendering function [
For statistical analysis purposes the following variables were created:
Any preoperative edema classifies whether or not a patient had any edema prior to surgery. Patients with no edema prior to surgery are not classified or described in any of the following variables due to the fact that their FLAIR signal change volume can only stay the same or increase (not decrease) Change in FLAIR signal change volume is the change from pre- to post-operative. This value is negative if the FLAIR signal change volume decreased and positive if it increased Percent change in FLAIR signal change volume is similar to change in FLAIR signal change volume (described above) only the magnitude of the change is given with respect to the baseline values Increase/decrease describes whether an increase or decrease in FLAIR signal change volume was observed as postoperative as compared to preoperative Complete resolution notes whether or not the patients FLAIR signal change volume was 0 at postoperative.
RESULTS
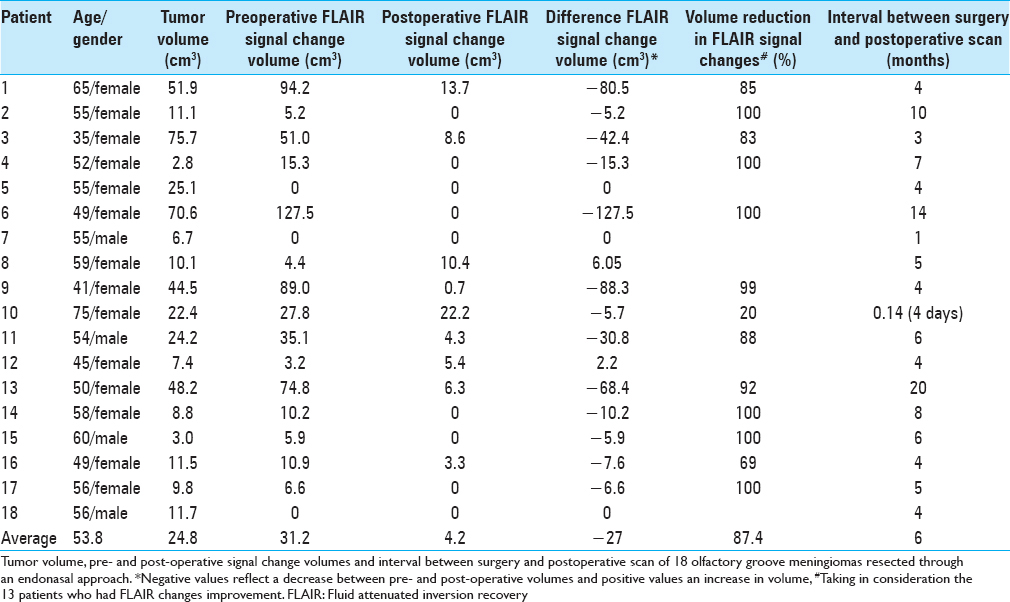
Individual assessment of tumor, pre- and post-operative FLAIR signal change volumes yielded the results displayed on
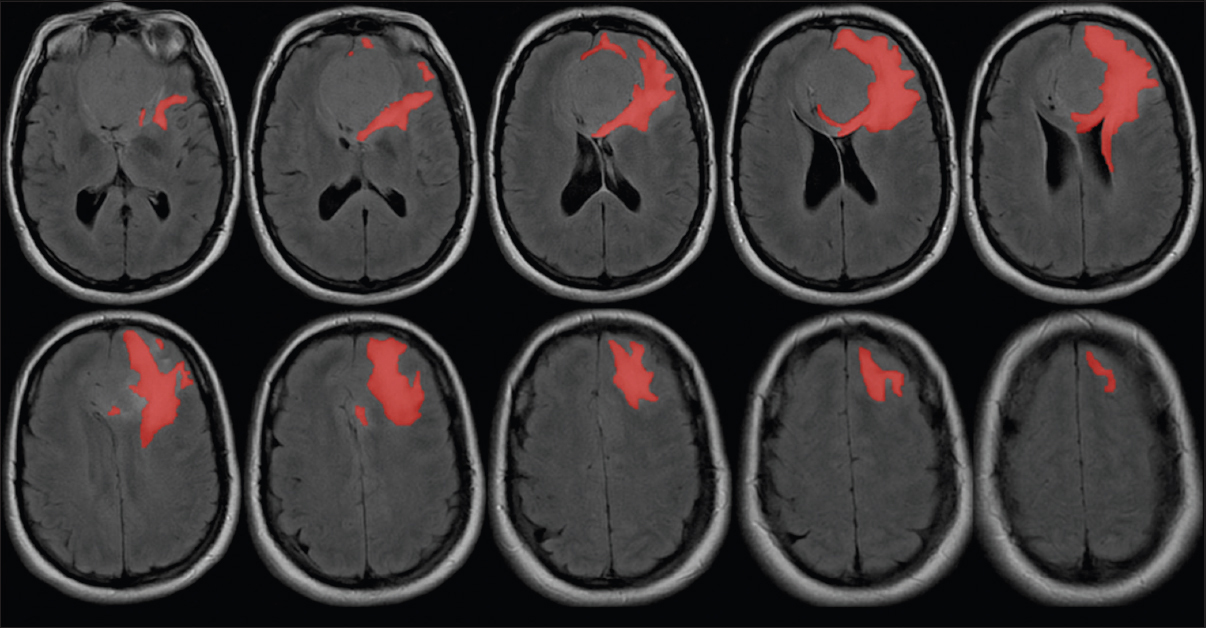
Only 2 (9%) of the 18 cases presented an increase in the postoperative edema volume when compared to the preoperative amount. These 2 patients with expansion in FLAIR changes had a volume rise of 2.2 cm3 and 6 cm3 respectively. In 3 (16.7%) of the 18 cases no preoperative FLAIR changes were observed and they were still absent in the postoperative MRI and other 6 (33.4%) patients had total resolution of the FLAIR changes. This represents a group of 9 patients (50%) with absolutely no FLAIR changes after endoscopic endonasal total resection of olfactory groove tumors. There were 13 patients who started with significant FLAIR change in the preoperative MRI and had documented a reduction in the FLAIR volume postoperatively; this reduction was of 87.4% of the volume on average.
Overall summarized data are presented on
As expected with the transcribiform approach, all of the 4 patients (22.2%) that presented with olfaction preoperatively became anosmic after surgery; the other patients were already anosmic prior to treatment and remained so afterwards. Due to the need for removal of the cribiform plate, the transcribiform approach, at least in its current configuration, does not allow for olfaction preservation.
The most common complication was postoperative cerebrospinal fluid (CSF) leakage (5 patients - 27.8%); these were more common during the early years (4 patients) of the development of the transcribiform approach and were significantly reduced with the implementation of the pedicled nasoseptal flap (1 patient). All these patients were brought back to the operating room for repositioning of the flap or augmentation of the skull base reconstruction. There was no postoperative meningitis, but 1 patient did present with a cerebral abscess after surgery, which was successfully managed with stereotactic aspiration and intravenous antibiotic therapy. There were no significant vascular events, ischemic or hemorrhagic. One patient [number 10,
DISCUSSION
Olfactory groove meningiomas are formidable lesions; given their location and often-insidious growth, they can reach sizeable dimensions, promoting compression of the surrounding frontal lobes, with significant behavioral and personality disorders. Compression of the olfactory bulbs frequently leads to anosmia; tumor progression posteriorly can lead to damage to the optic apparatus.
Due to their ability to slowly progress, it is not uncommon to find large lesions with impressive cerebral edema upon diagnosis. FLAIR sequence is one of the commonly utilized radiological techniques to detect and assess cerebral parenchymal changes due to edema and encephalomalacia; it basically nullifies signals from CSF while enhancing the visibility of fluid within the cerebral tissue itself. These features prompted the authors to elect it as a method for objective analysis of the volume of the edematous brain in the present series.
Several surgical techniques have been proposed for their transcranial removal;[
The last decade witnessed the rise and increasing popularity of endonasal endoscopic approaches in the treatment of a wide variety of ventral skull base pathologies.[
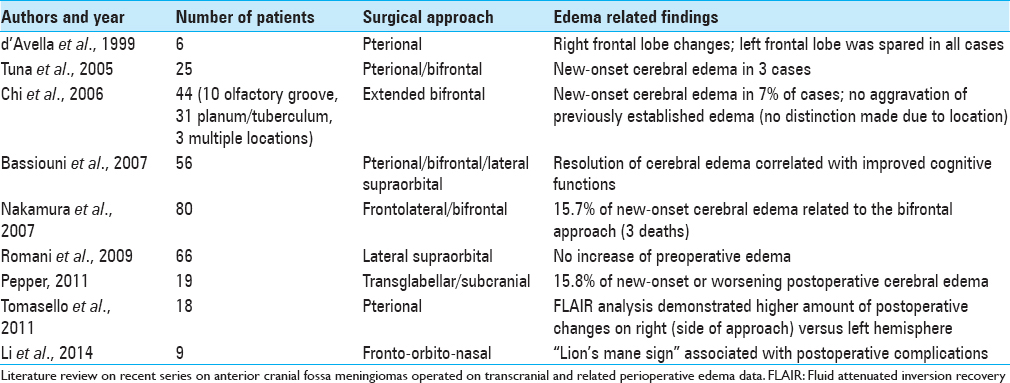
The endonasal resection of anterior cranial fossa meningiomas poses some potentially attractive features: Early tumor devascularization, wide removal of infiltrated, hyperostotic bone during the approach itself whilst promoting little to no brain manipulation are among them. The possibility of tumor removal without the need for brain retraction is particularly enticing in olfactory groove meningiomas, especially the large and giant tumors that impose themselves upon the frontal lobes and course with behavioral changes. Although several clinical series have described the surgical results of transcranial resection of these tumors, the literature is still somewhat scarce regarding the impact of these procedures on brain structure and subsequently, new onset or worsening of cerebral edema and encephalomalacia. In 1999 d’Avella et al.[
Clinical series describing the endonasal transcribiform resection of olfactory groove meningiomas are also scarce,[
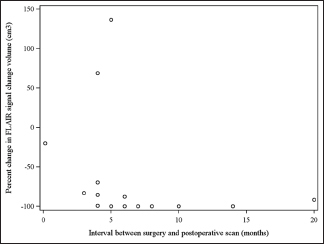
The authors acknowledge certain limitations in the present study: The lack of a control group, the relatively small patient sample, the heterogeneity of tumor volumes and the lack of uniformity regarding timing of the postoperative scans are noted. Given the retrospective design of the study there was little to be done regarding the latter; nonetheless, as shown in
To the author's knowledge, this is the first study to objectively document in a quantitative fashion the results of minimal to no brain retraction afforded by the endonasal corridor. Future, more comprehensive studies will be necessary to determine whether this feature impacts the clinical course of these patients and how it compares with transcranial approaches.
CONCLUSIONS
Endoscopic endonasal approaches appear to be a feasible method for total removal of select olfactory groove meningiomas. FLAIR signal changes tend to resolve after tumor resection and do not seem to worsen with this operative technique. Further comparative studies are necessary to determine whether this feature differs from open approaches and its impact on the clinical course of these patients.
ACKNOWLEDGMENTS
The authors thank Dr. Carl H. Snyderman and Dr. Paul A. Gardner, who have participated in the clinical management of some of these patients.
References
1. Aguiar PH, Tahara A, Almeida AN, Simm R, Silva AN, Maldaun MV. Olfactory groove meningiomas: Approaches and complications. J Clin Neurosci. 2009. 16: 1168-73
2. Attia M, Kandasamy J, Jakimovski D, Bedrosian J, Alimi M, Lee DL. The importance and timing of optic canal exploration and decompression during endoscopic endonasal resection of tuberculum sella and planum sphenoidale meningiomas. Neurosurgery. 2012. 71: 58-67
3. Babu R, Barton A, Kasoff SS. Resection of olfactory groove meningiomas: Technical note revisited. Surg Neurol. 1995. 44: 567-72
4. Bassiouni H, Asgari S, Stolke D. Olfactory groove meningiomas: Functional outcome in a series treated microsurgically. Acta Neurochir (Wien). 2007. 149: 109-21
5. Cappabianca P, Alfieri A, Thermes S, Buonamassa S, de Divitiis E. Instruments for endoscopic endonasal transsphenoidal surgery. Neurosurgery. 1999. 45: 392-5
6. Cappabianca P, Cavallo LM, Colao A, Del Basso De Caro M, Esposito F, Cirillo S. Endoscopic endonasal transsphenoidal approach: Outcome analysis of 100 consecutive procedures. Minim Invasive Neurosurg. 2002. 45: 193-200
7. Cappabianca P, Cavallo LM, de Divitiis O, Solari D, Esposito F, Colao A. Endoscopic pituitary surgery. Pituitary. 2008. 11: 385-90
8. Chi JH, Parsa AT, Berger MS, Kunwar S, McDermott MW. Extended bifrontal craniotomy for midline anterior fossa meningiomas: Minimization of retraction-related edema and surgical outcomes. Neurosurgery. 2006. 59: ONS426-33
9. Colli BO, Carlotti CG, Assirati JA, Santos MB, Neder L, Santos AC. Olfactory groove meningiomas: Surgical technique and follow-up review. Arq Neuropsiquiatr. 2007. 65: 795-9
10. d’Avella D, Salpietro FM, Alafaci C, Tomasello F. Giant olfactory meningiomas: The pterional approach and its relevance for minimizing surgical morbidity. Skull Base Surg. 1999. 9: 23-31
11. de Divitiis E, Cavallo LM, Esposito F, Stella L, Messina A. Extended endoscopic transsphenoidal approach for tuberculum sellae meningiomas. Neurosurgery. 2007. 61: 229-37
12. de Divitiis E, Esposito F, Cappabianca P, Cavallo LM, de Divitiis O. Tuberculum sellae meningiomas: High route or low route. A series of 51 consecutive cases?. Neurosurgery. 2008. 62: 556-63
13. de Divitiis E, Esposito F, Cappabianca P, Cavallo LM, de Divitiis O, Esposito I. Endoscopic transnasal resection of anterior cranial fossa meningiomas. Neurosurg Focus. 2008. 25: E8-
14. El-Bahy K. Validity of the frontolateral approach as a minimally invasive corridor for olfactory groove meningiomas. Acta Neurochir (Wien). 2009. 151: 1197-205
15. Fernandez-Miranda JC, Gardner PA, Prevedello DM, Kassam AB. Expanded endonasal approach for olfactory groove meningioma. Acta Neurochir (Wien). 2009. 151: 287-8
16. Frank G, Pasquini E, Doglietto F, Mazzatenta D, Sciarretta V, Farneti G. The endoscopic extended transsphenoidal approach for craniopharyngiomas. Neurosurgery. 2006. 59: ONS75-83
17. Frank G, Pasquini E. Endoscopic endonasal approaches to the cavernous sinus: Surgical approaches. Neurosurgery. 2002. 50: 675-
18. Frank G, Sciarretta V, Calbucci F, Farneti G, Mazzatenta D, Pasquini E. The endoscopic transnasal transsphenoidal approach for the treatment of cranial base chordomas and chondrosarcomas. Neurosurgery. 2006. 59: ONS50-7
19. Garcia-Navarro V, Lancman G, Guerrero-Maldonado A, Anand VK, Schwartz TH. Use of a side-cutting aspiration device for resection of tumors during endoscopic endonasal approaches. Neurosurg Focus. 2011. 30: E13-
20. Gardner PA, Kassam AB, Thomas A, Snyderman CH, Carrau RL, Mintz AH. Endoscopic endonasal resection of anterior cranial base meningiomas. Neurosurgery. 2008. 63: 36-52
21. Gazzeri R, Galarza M, Gazzeri G. Giant olfactory groove meningioma: Ophthalmological and cognitive outcome after bifrontal microsurgical approach. Acta Neurochir (Wien). 2008. 150: 1117-25
22. González-Darder JM, Pesudo-Martínez JV, Bordes-García V, Quilis-Quesada V, Talamantes-Escrivá F, González-López P. Olfactory groove meningiomas. Radical microsurgical treatment through the bifrontal approach. Neurocirugia (Astur). 2011. 22: 133-9
23. Hallacq P, Moreau JJ, Fischer G, Beziat JL. Frontal sinus approach to olfactory groove meningiomas. Neurochirurgie. 1999. 45: 329-37
24. Hallacq P, Moreau JJ, Fischer G, Béziat JL. Trans-sinusal frontal approach for olfactory groove meningiomas. Skull Base. 2001. 11: 35-46
25. Hassler W, Zentner J. Pterional approach for surgical treatment of olfactory groove meningiomas. Neurosurgery. 1989. 25: 942-5
26. Hassler W, Zentner J. Surgical treatment of olfactory groove meningiomas using the pterional approach. Acta Neurochir Suppl (Wien). 1991. 53: 14-8
27. Jho HD, Carrau RL. Endoscopic endonasal transsphenoidal surgery: Experience with 50 patients. J Neurosurg. 1997. 87: 44-51
28. Kassam A, Carrau RL, Snyderman CH, Gardner P, Mintz A. Evolution of reconstructive techniques following endoscopic expanded endonasal approaches. Neurosurg Focus. 2005. 19: E8-
29. Kassam A, Snyderman CH, Carrau RL, Gardner P, Mintz A. Endoneurosurgical hemostasis techniques: Lessons learned from 400 cases. Neurosurg Focus. 2005. 19: E7-
30. Kassam A, Snyderman CH, Mintz A, Gardner P, Carrau RL. Expanded endonasal approach: The rostrocaudal axis. Part I. Posterior clinoids to the foramen magnum. Neurosurg Focus. 2005. 19: E3-
31. Kassam A, Snyderman CH, Mintz A, Gardner P, Carrau RL. Expanded endonasal approach: The rostrocaudal axis. Part II. Posterior clinoids to the foramen magnum. Neurosurg Focus. 2005. 19: E4-
32. Kassam A, Thomas AJ, Snyderman C, Carrau R, Gardner P, Mintz A. Fully endoscopic expanded endonasal approach treating skull base lesions in pediatric patients. J Neurosurg. 2007. 106: 75-86
33. Kassam AB, Gardner P, Snyderman C, Mintz A, Carrau R. Expanded endonasal approach: Fully endoscopic, completely transnasal approach to the middle third of the clivus, petrous bone, middle cranial fossa, and infratemporal fossa. Neurosurg Focus. 2005. 19: E6-
34. Kassam AB, Gardner PA, Snyderman CH, Carrau RL, Mintz AH, Prevedello DM. Expanded endonasal approach, a fully endoscopic transnasal approach for the resection of midline suprasellar craniopharyngiomas: A new classification based on the infundibulum. J Neurosurg. 2008. 108: 715-28
35. Kassam AB, Prevedello DM, Carrau RL, Snyderman CH, Gardner P, Osawa S. The front door to meckel's cave: An anteromedial corridor via expanded endoscopic endonasal approach - Technical considerations and clinical series. Neurosurgery. 2009. 64: ons71-82
36. Kassam AB, Prevedello DM, Carrau RL, Snyderman CH, Thomas A, Gardner P. Endoscopic endonasal skull base surgery: Analysis of complications in the authors’ initial 800 patients. J Neurosurg. 2011. 114: 1544-68
37. Kassam AB, Thomas A, Carrau RL, Snyderman CH, Vescan A, Prevedello D. Endoscopic reconstruction of the cranial base using a pedicled nasoseptal flap. Neurosurgery. 2008. 63: ONS44-52
38. Komotar RJ, Starke RM, Raper DM, Anand VK, Schwartz TH. Endoscopic endonasal versus open transcranial resection of anterior midline skull base meningiomas. World Neurosurg. 2012. 77: 713-24
39. Koutourousiou M, Fernandez-Miranda JC, Stefko ST, Wang EW, Snyderman CH, Gardner PA. Endoscopic endonasal surgery for suprasellar meningiomas: Experience with 75 patients. J Neurosurg. 2014. 120: 1326-39
40. Kunicki A, Uhl A. The clinical picture and results of treatment in 26 cases of olfactory groove meningiomas. Acta Med Pol. 1970. 11: 103-17
41. Li MS, Portman SM, Rahal A, Mohr G, Balasingam V. The lion's mane sign: Surgical results using the bilateral fronto-orbito-nasal approach in large and giant anterior skull base meningiomas. J Neurosurg. 2014. 120: 315-20
42. Liang RS, Zhou LF, Mao Y, Zhang R, Yang WZ. Microsurgical removal of olfactory groove meningiomas. Zhonghua Zhong Liu Za Zhi. 2011. 33: 70-5
43. Liu JK, Christiano LD, Patel SK, Tubbs RS, Eloy JA. Surgical nuances for removal of olfactory groove meningiomas using the endoscopic endonasal transcribriform approach. Neurosurg Focus. 2011. 30: E3-
44. Liu Y, Liu M, Chen Y, Li F, Wang H, Zhu S. Microsurgical total removal of olfactory groove meningiomas and reconstruction of the invaded skull bases. Int Surg. 2007. 92: 167-73
45. Mayfrank L, Gilsbach JM. Interhemispheric approach for microsurgical removal of olfactory groove meningiomas. Br J Neurosurg. 1996. 10: 541-5
46. McLaughlin N, Ditzel Filho LF, Prevedello DM, Kelly DF, Carrau RL, Kassam AB. Side-cutting aspiration device for endoscopic and microscopic tumor removal. J Neurol Surg B Skull Base. 2012. 73: 11-20
47. Nakamura M, Struck M, Roser F, Vorkapic P, Samii M. Olfactory groove meningiomas: Clinical outcome and recurrence rates after tumor removal through the frontolateral and bifrontal approach. Neurosurgery. 2007. 60: 844-52
48. Ottenhausen M, Banu MA, Placantonakis DG, Tsiouris AJ, Khan OH, Anand VK. Endoscopic endonasal resection of suprasellar meningiomas: The importance of case selection and experience in determining extent of resection, visual improvement, and complications. World Neurosurg. 2014. 82: 442-9
49. Pepper JP, Hecht SL, Gebarski SS, Lin EM, Sullivan SE, Marentette LJ. Olfactory groove meningioma: Discussion of clinical presentation and surgical outcomes following excision via the subcranial approach. Laryngoscope. 2011. 121: 2282-9
50. Poppen JL. Operative techniques for removal of olfactory groove and suprasellar meningiomas. Clin Neurosurg. 1964. 11: 1-7
51. Romani R, Lehecka M, Gaal E, Toninelli S, Celik O, Niemelä M. Lateral supraorbital approach applied to olfactory groove meningiomas: Experience with 66 consecutive patients. Neurosurgery. 2009. 65: 39-52
52. Rubin G, Ben David U, Gornish M, Rappaport ZH. Meningiomas of the anterior cranial fossa floor. Review of 67 cases. Acta Neurochir (Wien). 1994. 129: 26-30
53. Spektor S, Valarezo J, Fliss DM, Gil Z, Cohen J, Goldman J. Olfactory groove meningiomas from neurosurgical and ear, nose, and throat perspectives: Approaches, techniques, and outcomes. Neurosurgery. 2005. 57: 268-80
54. Tamaki N, Yin D. Giant olfactory groove meningiomas: Advantages of the bilateral fronto-orbitonasal approach. J Clin Neurosci. 1999. 6: 302-05
55. Tomasello F, Angileri FF, Grasso G, Granata F, De Ponte FS, Alafaci C. Giant olfactory groove meningiomas: Extent of frontal lobes damage and long-term outcome after the pterional approach. World Neurosurg. 2011. 76: 311-7
56. Tuna H, Bozkurt M, Ayten M, Erdogan A, Deda H. Olfactory groove meningiomas. J Clin Neurosci. 2005. 12: 664-8
57. Turazzi S, Cristofori L, Gambin R, Bricolo A. The pterional approach for the microsurgical removal of olfactory groove meningiomas. Neurosurgery. 1999. 45: 821-5
58. Van Gompel JJ, Frank G, Pasquini E, Zoli M, Hoover J, Lanzino G. Expanded endonasal endoscopic resection of anterior fossa meningiomas: Report of 13 cases and meta-analysis of the literature. Neurosurg Focus. 2011. 30: E15-