- Department of Neurosurgery, Narayana Health, Bengaluru, Karnataka, India
- Department of Neurosurgery, Chinmaya Mission Hospital, Bengaluru, Karnataka, India
- Department of Neurosurgery, Narayana Health Mazumdar Shaw Medical Center, Bengaluru, Karnataka, India
- Department of Neurosurgery, Neuro Health Foundation Clinic, Borivali West, Mumbai, Maharashtra, India
- Department of Neurosurgery, Apollo Hospitals, Chennai, Tamil Nadu, India.
Correspondence Address:
Mrudul Mohinish Bhatjiwale, Department of Neurosurgery, Narayana Health, Bengaluru, Karnataka, India.
DOI:10.25259/SNI_391_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Mrudul Mohinish Bhatjiwale1, Kiran Mariswamappa2, Komal Prasad Chandrachari3, Mohinish Bhatjiwale4, Tanvi Joshi5, Thimappa Hegde3, Akshay Vijay Kulkarni3. Malignant bihemispheric cerebral edema after cranioplasty – An extension of the Monro-Kellie doctrine and predictive factors. 04-Aug-2023;14:271
How to cite this URL: Mrudul Mohinish Bhatjiwale1, Kiran Mariswamappa2, Komal Prasad Chandrachari3, Mohinish Bhatjiwale4, Tanvi Joshi5, Thimappa Hegde3, Akshay Vijay Kulkarni3. Malignant bihemispheric cerebral edema after cranioplasty – An extension of the Monro-Kellie doctrine and predictive factors. 04-Aug-2023;14:271. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=12489
Abstract
Background: Several changes in normal pressure dynamics on the brain occur with a decompressive craniectomy and subsequent cranioplasty. Dead space volume is an important factor contributing to intracranial volume postcranioplasty. A decrease in this volume due to negative suction drain along with relative negative pressure on the brain with the loss of external atmospheric pressure may lead to fatal cerebral edema.
Case Description: A 52-year-old gentleman with a 210 mL volume and middle cerebral artery territory infarction underwent an emergency craniectomy and 6 months later a titanium mold cranioplasty. Precranioplasty computed tomography (CT) scan evaluation revealed a sunken skin flap with a 9 mm contralateral midline shift. Immediately following an uneventful surgery, the patient had sudden fall in blood pressure to 60/40 mmHg and over a few min had dilated fixed pupils. CT revealed severe diffuse cerebral edema in bilateral hemispheres with microhemorrhages and expansion of the sunken right gliotic brain along with ipsilateral ventricular dilatation. Despite undergoing a contralateral decompressive craniectomy due to the midline shift toward the right, the outcome was fatal.
Conclusion: Careful preoperative risk assessment in cranioplasty and close monitoring postprocedure is crucial, especially in malnourished, poststroke cases, with a sinking skin flap syndrome, and a long interval between decompressive craniectomy and cranioplasty. Elective preventive measures and a low threshold for CT scanning and removal of the bone flap or titanium mold are recommended.
Keywords: Craniectomy, Complication, Cranioplasty, Edema, Malignant, Monro-Kellie, Trephination
INTRODUCTION
Cranioplasty in literature dates back to the 16th century where Fallopius makes mention of a gold plate used to reconstruct a skull defect, though there is a scattered evidence of similar procedures being performed since the Incans.[
Postcranioplasty rebound cerebral edema, initially described as “pseudohypoxic brain swelling” by Van Roost D et al.,[
We report a case of malignant cerebral edema with multiple ipsilateral hemorrhagic foci occurring within an hour of a titanium mold cranioplasty procedure. The aim of this manuscript is to bring to attention a rare, often under-rated complication of a seemingly straightforward procedure, elucidate possible pathophysiological mechanisms, and identify patients at a high risk for the same.
CASE REPORT
Initial evaluation and intervention
A 52-year-old gentleman had presented to the emergency room with sudden onset left hemiparesis, with a Glasgow Coma Scale (GCS) of E2M5Vt. He was diagnosed with a 210 mL volume, middle cerebral artery (MCA) territory infarction on magnetic resonance (MR) imaging with mass effect and a 12 mm midline shift. He was also diagnosed with a right brachial artery embolus on routine large vessel and neck MR angiograms. Carotid vasculature was noted to be normal with no narrowing or filling defect. An emergency right fronto-temporo-parietal decompressive with lax duraplasty was performed followed by a right brachial embolectomy by the vascular team. Further evaluation with a cardiac 2D echogram revealed no regional wall motion abnormality with mild concentric left ventricular hypertrophy (LVH), normally functioning valves and no clots or vegetations. Postoperatively, he had an uneventful recovery and over a week GCS improved to E4M6V5. He was discharged on therapeutic doses of low molecular weight heparin for the brachial artery embolus.
Cranioplasty
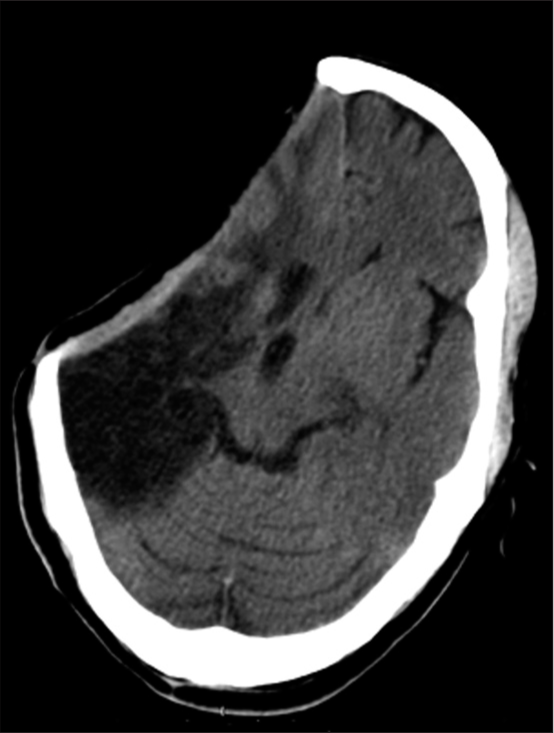
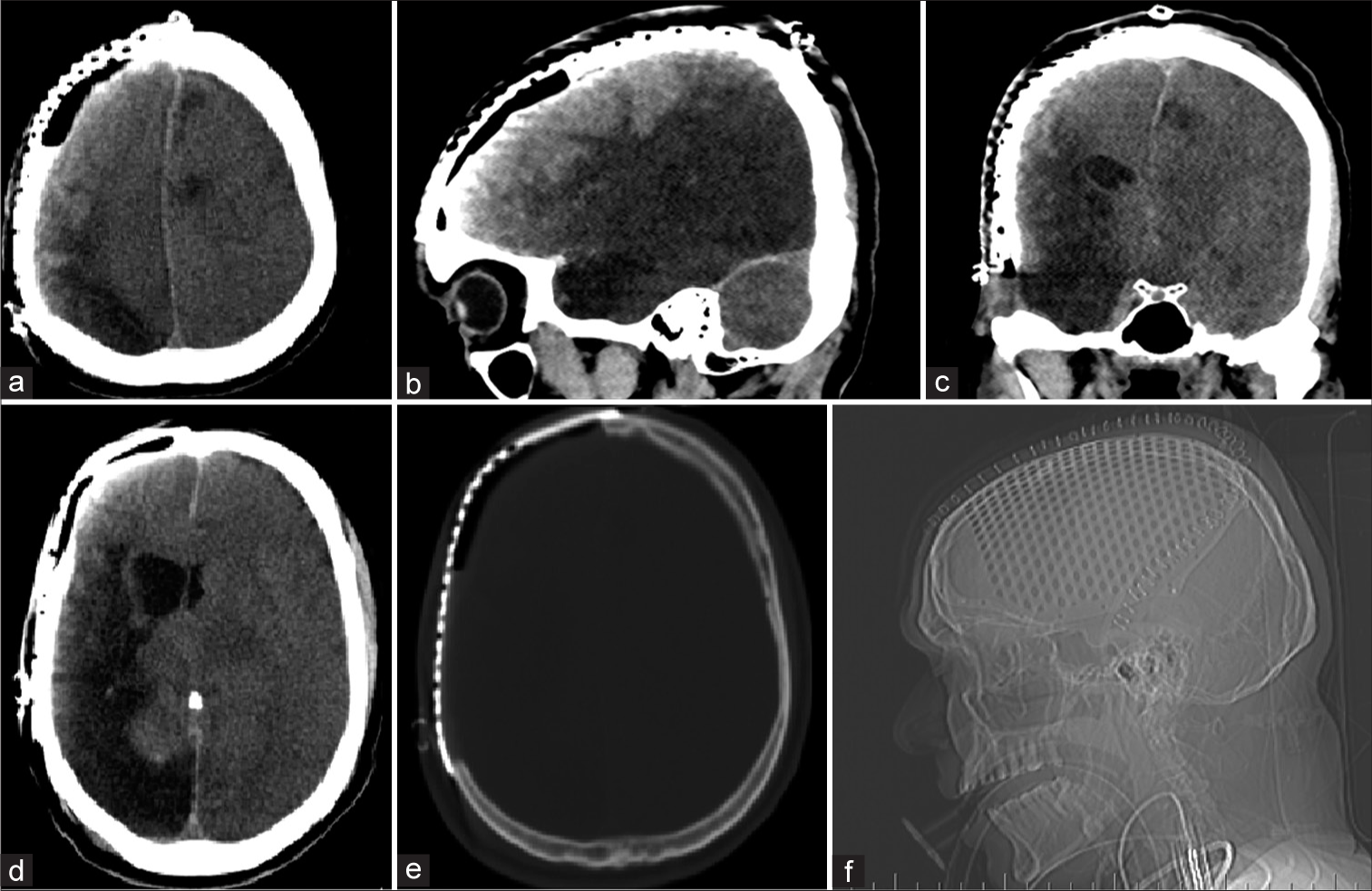
He presented 6 months later for a cranioplasty. He was conscious alert and oriented with modified Ashworth Grade II hypertonia and Medical Research Council Grade 3 power in the left upper and lower limbs. A computed tomography (CT) scan revealed a sunken skin flap with a 9 mm midline shift to the left along with gliotic changes in the right MCA territory [
Postoperative period and outcome
The patient did not wake from anesthesia and had sudden fall in blood pressure (BP) to 60/40 mmHg a few minutes after shifting, on ventilator, to the intensive care unit. The hypotension lasted for less than a minute and he was stabilized with inotropes and noradrenaline boluses and later a drip infusion, with close titration owing to a very labile BP highly sensitive to minor adjustments in the inotropes. Blood gases, electrolytes, and postoperative hematocrit were within normal limits. A screening echocardiogram at the time showed an ejection fraction of 55% with freely moving valves and no clots or vegetations, ruling out a cardiac cause for the hypotension. During and immediately following stabilization, his pupils began dilating bilaterally from 2 mm to 8 mm over 30 min.
A CT scan showed diffuse severe cerebral edema in bilateral hemispheres with effaced basal cisterns, microhemorrhages, and expansion of the sunken right gliotic brain along with ipsilateral ventricular dilatation. There was poor grey-white matter differentiation in the contralateral (left) side with a midline shift of 5 mm toward the right (cranioplasty) side [
A magnetic resonance imaging was performed the subsequent day that showed bilateral posterior cerebral artery territory and brainstem infarcts [
Figure 3:
Post procedure magnetic resonance imaging revealed bilateral posterior circulation hemorrhagic infarcts and severe edema. Diffusion-weighted imaging showing diffusion restricting acute infarcts (a and b), decreased apparent diffusion coefficient (c), and blooming on gradient echo sequence (d).
Despite all possible efforts and interventions, the patient did not improve, had absent brainstem signs with dilated fixed pupils and a GCS of E1M1Vt, and eventually a fatal outcome.
Possible etiologies
In this case, other possibilities of malignant cerebral edema include fresh emboli from the heart causing fresh infarcts. Although fresh infarcts developing severe edema rapidly within an hour is rare, a screening cardiac 2D echogram was done at the time which showed normal findings apart from mild concentric LVH, and an ejection fraction of 55%, matching the evaluation done at the time of the initial infarct.
Hypoxia during surgery could theoretically cause bihemispheric edema; however, no such hypoxia or desaturation occurred intraoperatively or immediately postoperatively.
Hypotension due to an unrelated cause and subsequent hypoxic brain injury leading to malignant edema may be considered. However, no other systemic causes of hypotension could be identified. There was minimal blood loss during surgery and an 80–150 mL/h urine output throughout ruling out hypovolemia. Cardiac causes had been ruled out (as mentioned earlier) and there was no cause for septic shock immediate postoperatively. It was hence concluded that the hypotension was a consequence of severe brain edema and loss of autonomic control, further supported by the lability of the BP and extreme sensitivity to inotropic agents.
After excluding the above, the sequence of events suggests postcranioplasty malignant edema due to a change in pressure dynamics, as the most likely cause of the deterioration.
DISCUSSION
Postcranioplasty cerebral edema is, fortunately, an exceedingly rare complication, with twenty odd cases reported throughout world literature till date,[
Pathophysiology
When a decompressive craniectomy is performed, the Monro-Kellie doctrine[
k (constant) = Brain parenchymal volume + Cerebral blood volume + Cerebrospinal fluid volume + Dead space volume
This dead space has a natural relative negative pressure[
In a gliotic brain, the parenchymal volume is already considerably reduced, and with cerebrospinal fluid (CSF) volume being relatively constant in a hyperacute setting, cerebral blood volume increases with decreasing dead space volume. An already dysfunctional cerebral microcirculation (due to trauma/infarction etc.) gives way to increased cerebral blood flow, leading to malignant edema and hemorrhages.
This is supported by the largest reported case series by Sviri[
Sviri also noted all patients had a closed suction drainage.[
Risk factors and predictors
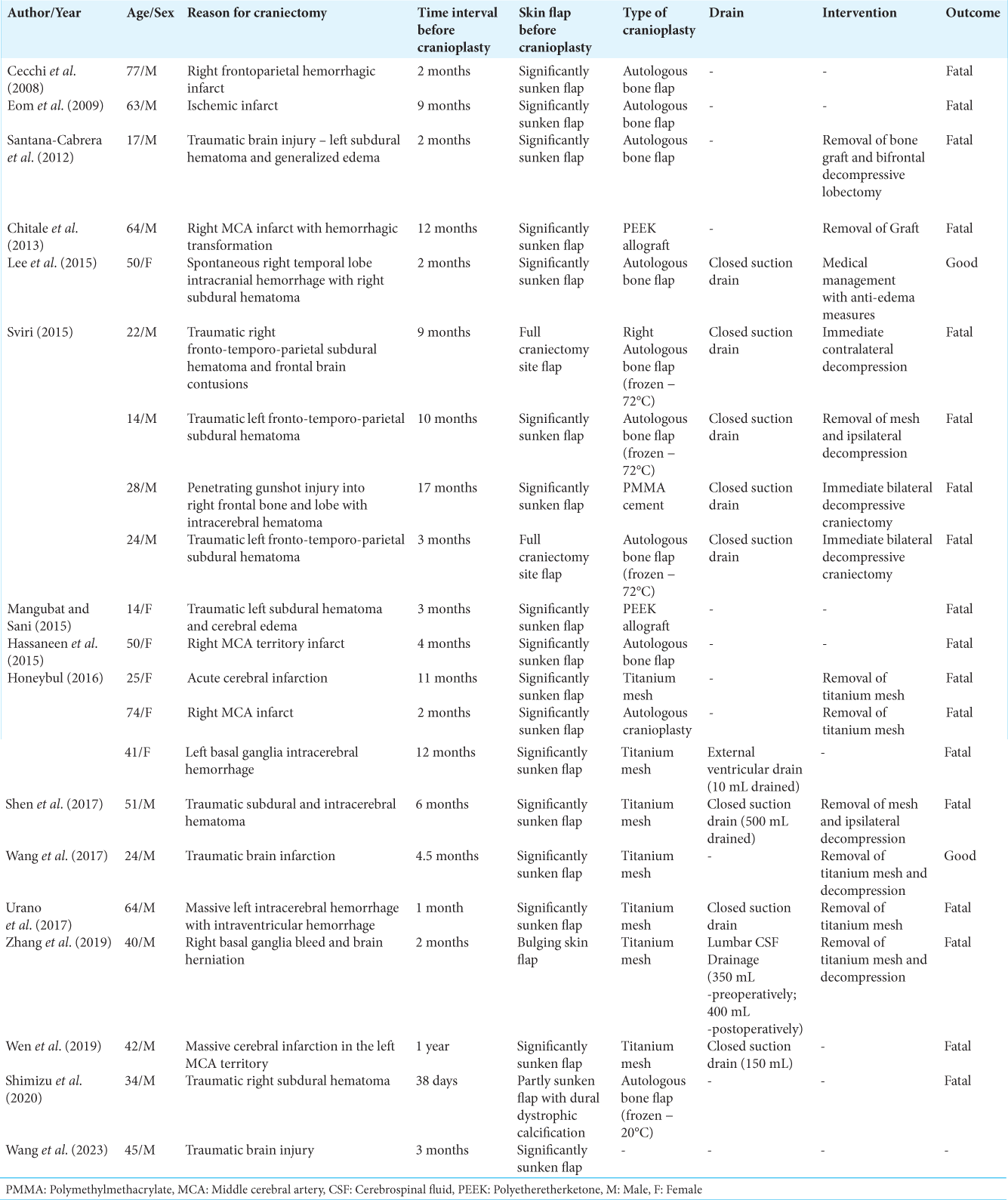
This complication occurred over all age groups and no particular age group could be identified as being at a high risk. Based on the reported cases in the literature till date, the following high-risk factors have been identified – Sinking skin flap syndrome, the time since craniectomy, and the primary pathology [
The above factors need validation and testing in large numbers of cranioplasty patients and must be suitably extended/modified and condensed into a risk scoring system, before being conclusively used to identify patients at a high risk of developing cerebral edema postcranioplasty.
Possible prevention in high-risk patients postoperatively
As per the extended Monro-Kellie doctrine described above, we believe that minimization of either the dead space or the negative pressure in it[
Generous hydration preoperatively to promote CSF formation and decrease shear on the brain parenchyma in accordance with the extended Monro-Kellie doctrine A slow spinal infusion of normal saline through a lumbar puncture to expand the compressed cerebral hemisphere[ Moderate irrigation and partly filling saline in the dead space – As saline is slowly absorbed/drained, it may potentially help in preventing sudden brain shifts. Caution must be exercised in not filling excess saline that may exert pressure on the brain and accentuate the preexisting midline shift Dead space may be reduced by dependent positioning of the patient with the ipsilateral side gravity dependent (like in the management for sinking skin flap syndrome) after the procedure Passive subgaleal drain – without suction.
CONCLUSION
Decompressive craniectomy changes the intracranial milieu and adds external atmospheric pressure to the myriad of forces at play on the brain. The modified Monro-Kellie doctrine as described emphasizes the role of dead space volume after cranioplasty, which we believe plays a critical role in malignant cerebral edema after cranioplasty. It is crucial to assess the risk of this fatal complication preprocedure and take steps to prevent it in the high-risk subgroup. Trephination syndrome, an etiology of traumatic brain injury and cranioplasty within 3 months after craniectomy, is high-risk factors. In these patients, close monitoring and measures to prevent cerebral edema are recommended.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Publication of this article was made possible by the James I. and Carolyn R. Ausman Educational Foundation.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Aa L, Wj G. Chronic subdural hematoma; expansion of compressed cerebral hemisphere and relief of hypotension by spinal injection of physiologic saline solution. N Engl J Med. 1948. 239: 493-6
2. Acciarri N, Nicolini F, Martinoni M. Cranioplasty: Routine surgical procedure or risky operation?. World J Surg Res. 2016. 9: 5
3. Ashayeri K, Jackson EM, Huang J, Brem H, Gordon CR. Syndrome of the trephined: A systematic review. Neurosurgery. 2016. 79: 525-34
4. Bhaskar IP, Zaw NN, Zheng M, Lee GY. Bone flap storage following craniectomy: A survey of practices in major Australian Neurosurgical centres. ANZ J Surg. 2011. 81: 137-41
5. Broughton E, Pobereskin L, Whitfield PC. Seven years of cranioplasty in a regional neurosurgical centre. Br J Neurosurg. 2014. 28: 34-9
6. Cecchi PC, Rizzo P, Campello M, Schwarz A. Haemorrhagic infarction after autologous cranioplasty in a patient with sinking flap syndrome. Acta Neurochir (Wien). 2008. 150: 409-10
7. Chitale R, Tjoumakaris S, Gonzalez F, Dumont AS, Rosenwasser RH, Jabbour PM. Infratentorial and supratentorial strokes after a cranioplasty. Neurologist. 2013. 19: 17-21
8. Eom KS, Kim DW, Kang SD. Bilateral diffuse intracerebral hemorrhagic infarction after cranioplasty with autologous bone graft. Clin Neurol Neurosurg. 2010. 112: 336-40
9. Hassaneen W, Germanwala A, Tsimpas A. Malignant cerebral edema following cranioplasty. J Clin Neurosci. 2016. 25: 130-2
10. Honeybul S, Damodaran O, Lind CR, Lee G. Malignant cerebral swelling following cranioplasty. J Clin Neurosci. 2016. 29: 3-6
11. Honeybul S. Complications of decompressive craniectomy for head injury. J Clin Neurosci. 2010. 17: 977
12. Honeybul S. Sudden death following cranioplasty: A complication of decompressive craniectomy for head injury. Br J Neurosurg. 2011. 25: 345-5
13. Honeybul S. Sudden death following cranioplasty: Autoregulatory failure?. J Neurosurg. 2016. 124: 885-7
14. Lee GS, Park SQ, Kim R, Cho SJ. Unexpected severe cerebral edema after cranioplasty: Case report and literature review. J Korean Neurosurg Soc. 2015. 58: 76-8
15. Mangubat E, Sani S. Acute global ischemic stroke after cranioplasty case report and review of the literature. Neurologist. 2015. 19: 135-9
16. Mokri B. The Monro-kellie hypothesis: Applications in CSF volume depletion. Neurology. 2001. 56: 1746-8
17. Reid Gooch M, Gin GE, Kenning TJ, German JW. Complications of cranioplasty following decompressive craniectomy: Analysis of 62 cases. Neurosurg Focus. 2009. 26: E9
18. Sanan A, Haines SJ. Repairing holes in the head: A history of cranioplasty. Neurosurgery. 1997. 40: 588-603
19. Santana-Cabrera L, Sánchez-Palacios M, Pérez-Ortiz C, Rodríguez-Escot C. Massive postoperative cerebral swelling following cranioplasty. Int J Crit Illn Inj Sci. 2012. 2: 107-8
20. Shen L, Zhou Y, Xu J, Su Z. Malignant cerebral swelling after cranioplasty: Case report and literature review. World Neurosurg. 2018. 110: 4-10
21. Shimizu Y, Tsuchiya K, Fujisawa H. Cerebral swelling caused by deep venous thrombosis immediately after cranioplasty. Br J Neurosurg. 2023. 37: 907-10
22. Sviri GE. Massive cerebral swelling immediately after cranioplasty, a fatal and unpredictable complication: Report of 4 cases. J Neurosurg. 2015. 123: 11888-93
23. Ullah S, Khan N, Zeb H, Tahir H, Suri J. 2008 Sinking skin flap syndrome: Phenomenon of neurologic deterioration after decompressive craniectomy. Crit Care Med. 2016. 44: 577
24. Urano Y. Fatal cerebral swelling immediately after cranioplasty: A case report. Surg Neurol Int. 2017. 8: 156
25. Van Roost D, Thees C, Brenke C, Oppel F, Winkler PA, Schramm J. Pseudohypoxic brain swelling: A newly defined complication after uneventful brain surgery, probably related to suction drainage. Neurosurgery. 2003. 53: 1315-26
26. Wang H, Li W, Zhou L, Fu S, Chen B, Zhang S. Malignant cerebral swelling after cranioplasty due to ipsilateral intracranial vasculopathy: Case report and literature review. World Neurosurg. 2017. 107: 1044.e11-7
27. Wang S, Luan Y, Peng T, Wang G, Zhou L, Wu W. Malignant cerebral edema after cranioplasty: A case report and literature review. Brain Inj. 2023. p. 1-7
28. Wen G, Zeng P, Zhou J, Wang G, Wu G, Zeng W. Unexpected intracranial hemorrhage and death after cranioplasty in a patient with massive hemispheric infarction. J Craniofac Surg. 2019. 30: e378-80
29. Zanaty M, Chalouhi N, Starke RM, Clark SW, Bovenzi CD, Saigh M. Complications following cranioplasty: Incidence and predictors in 348 cases. J Neurosurg. 2015. 123: 182-8
30. Zhang X, Pan B, Ye Z, Li Z, Mo F, Wang X. Massive brain swelling after cranioplasty: A case report. J Neurol Surg A Cent Eur Neurosurg. 2019. 80: 498-502