- Department of Radiation Oncology, The Ohio State University, Columbus, Ohio,
- Department of Radiation Oncology, Emory University, Atlanta, Georgia,
- Department of Neuropathology, The Ohio State University, Columbus, Ohio.
- Department of Neurosurgery, The Ohio State University, Columbus, Ohio.
Correspondence Address:
Raju R. Raval, MD, DPhil, Department of Radiation Oncology, The Ohio State University, Columbus, Ohio, United States.
DOI:10.25259/SNI_827_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Sasha Beyer1, Nikhil T. Sebastian2, Rahul Neal Prasad1, Jacqueline Chu1, Kevin Liu1, Kajal Madan1, William Jiang1, Jayeeta Ghose1, Dukagjin M. Blakaj1, Joshua D. Palmer1, Mostafa Eltobgy3, Jose Otero3, James B. Elder4, Raju R. Raval1. Malignant ossifying fibromyxoid tumor of the brain treated with post-operative fractionated stereotactic radiation therapy: A case report and literature review. 30-Nov-2021;12:588
How to cite this URL: Sasha Beyer1, Nikhil T. Sebastian2, Rahul Neal Prasad1, Jacqueline Chu1, Kevin Liu1, Kajal Madan1, William Jiang1, Jayeeta Ghose1, Dukagjin M. Blakaj1, Joshua D. Palmer1, Mostafa Eltobgy3, Jose Otero3, James B. Elder4, Raju R. Raval1. Malignant ossifying fibromyxoid tumor of the brain treated with post-operative fractionated stereotactic radiation therapy: A case report and literature review. 30-Nov-2021;12:588. Available from: https://surgicalneurologyint.com/surgicalint-articles/11253/
Abstract
Background: Ossifying fibromyxoid tumor (OFMT) is a rare musculoskeletal soft-tissue neoplasm of uncertain histogenesis most frequently occurring in the lower extremities. Conventionally, considered benign, these tumors are often managed by surgical resection followed by surveillance. However, malignant OFMTs with an increased propensity for local recurrence and distant metastasis have been recently identified, and the role of adjuvant therapy in these more aggressive cases is unclear.
Case Description: We present, to the best of our knowledge, the first reported case of a primary, malignant, and intracranial OFMT. A 29-year-old female presented with recurrent headaches secondary to a large mass in her right frontal lobe. She underwent gross total resection of the brain mass with final pathology consistent with malignant OFMT demonstrating high-risk features including increased cellularity, grade, and mitotic activity. Due to these high-risk features, she received postoperative fractionated stereotactic radiation therapy (FSRT) to the resection cavity, and to the best of our knowledge, she represents the only known patient with OFMT to be treated with adjuvant FSRT. She tolerated the adjuvant treatment well with no acute or late toxicities and remains disease-free over 5 ½ years after resection.
Conclusion: Adjuvant FSRT appears to be a safe and efficacious approach for managing this rare intracranial disease presentation. We review this patient’s clinical course in the context of the literature to demonstrate the difficulties associated with accurate diagnosis of this rare tumor and the controversial role of adjuvant therapy in preventing disease recurrence in this patient population.
Keywords: Adjuvant therapy, Malignant intracranial ossifying fibromyxoid tumor, Malignant ossifying fibromyxoid tumor, Radiation therapy, Stereotactic radiotherapy
INTRODUCTION
Ossifying fibromyxoid tumor (OFMT) is a rare musculoskeletal soft-tissue neoplasm of uncertain histogenetic lineage.[
Although OFMTs have traditionally been considered to be benign, variants with a relatively high propensity for local recurrence and distant metastasis have more recently been reported in the literature.[
CASE DESCRIPTION
A previously healthy 29-year-old female presented to our institution with recurrent headaches, which progressively worsened over 2–3 months and were refractory to symptom-directed medical management. Through an interpreter, she reported headaches radiating from her occipital to frontal area and worsened in the mornings. She also reported a 2-week history of intermittent dizziness and nocturnal fevers. On presentation to the emergency department, she experienced an episode of vomiting. Physical exam confirmed a history of chronic hearing loss and use of hearing aids; however, no other neurological deficits were noted.
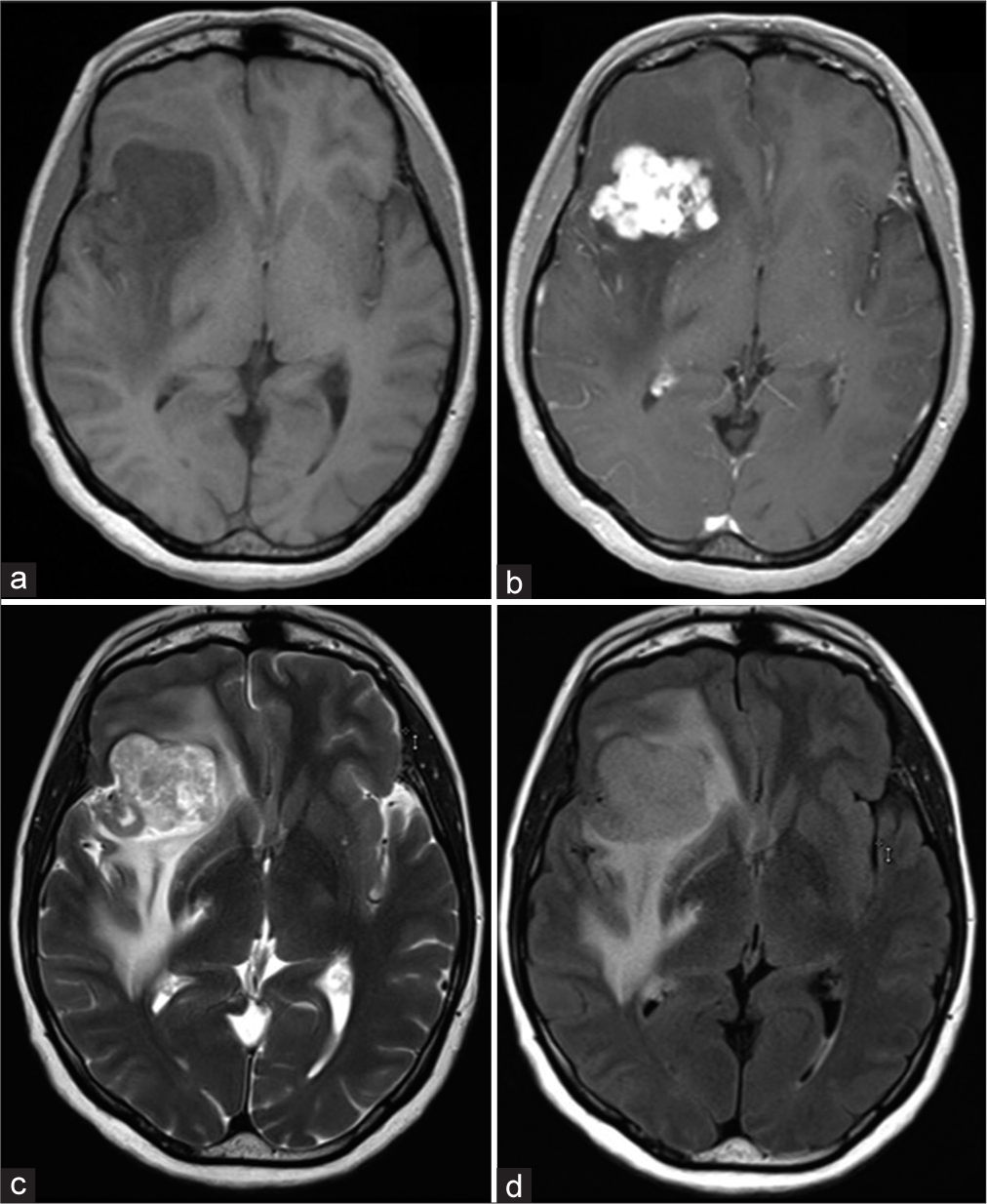
CT imaging of the head showed a peripherally hyperdense, centrally hypodense lesion in the right frontal lobe measuring 1.4 × 1.2 cm. Additional lesions were not readily apparent on the non-contrast CT image. Subsequent brain MRI with and without contrast showed a large, lobulated, enhancing mass in the right inferior frontal lobe with extensive surrounding edema. The mass measured 4.1 × 3.5 × 4.2 cm in size. A T1-weighted MRI sequence showed a hypointense mass [
Figure 1:
Brain MRI with and without contrast demonstrated a 4.2 cm lobulated, enhancing mass in the right inferior frontal lobe with extensive surrounding edema. The mass appears hypointense on the T1-weighted MRI non-contrast sequence (a) with diffuse enhancement on the post-contrast sequence (b). The extent of edema is noted on T2-weighted (c) and T2/FLAIR (d) images.
She underwent a gross total neurosurgical resection. The well-circumscribed superficial part of the tumor was sent for frozen section and was consistent with a low-grade neoplasm of unknown etiology. The tumor was dissected away from the surrounding brain in multiple specimens (piecemeal). No residual tumor was noted in the resection cavity by the neurosurgeon after completion of the procedure. Postsurgical brain MRI confirmed the absence of residual tumor in the right frontal resection cavity. The patient recovered after surgery with no complications.
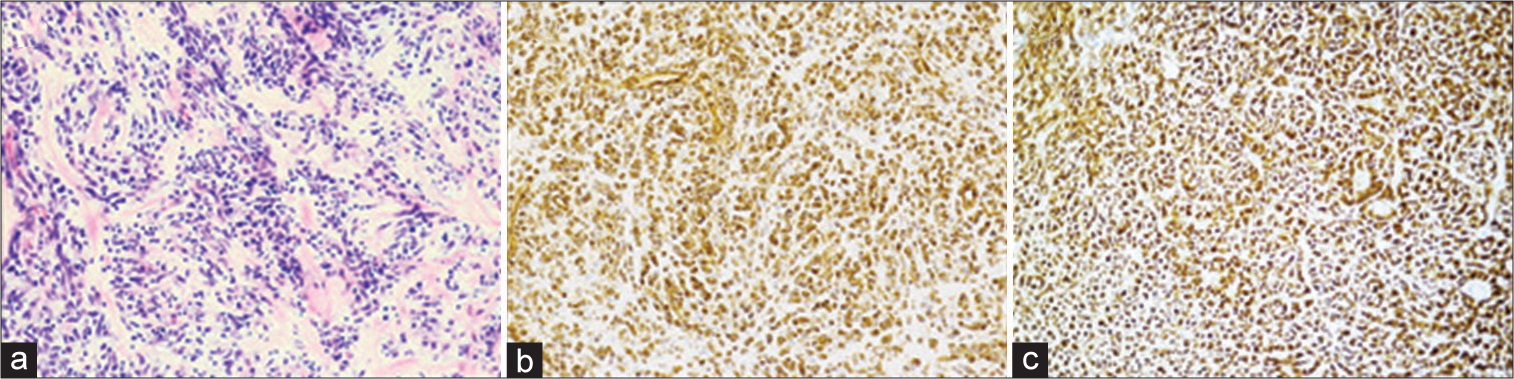
Final pathology showed a non-infiltrating neoplasm composed of lobules of round to spindle-shaped cells arranged in cords among a myxoid and collagenous matrix [
Figure 2:
Pathology revealed a well marginated neoplasm composed of lobules of round to spindle-shaped cells arranged in nests and cords among a myxoid and collagenous matrix surrounded by a peripheral rim of bone. Hematoxylin and eosin staining demonstrating lobules of spindle-shaped cells (×20) (a). Strong, diffuse immunohistochemical staining for vimentin (×20) (b). Diffuse immunohistochemical staining for CD56 (×20) (c).
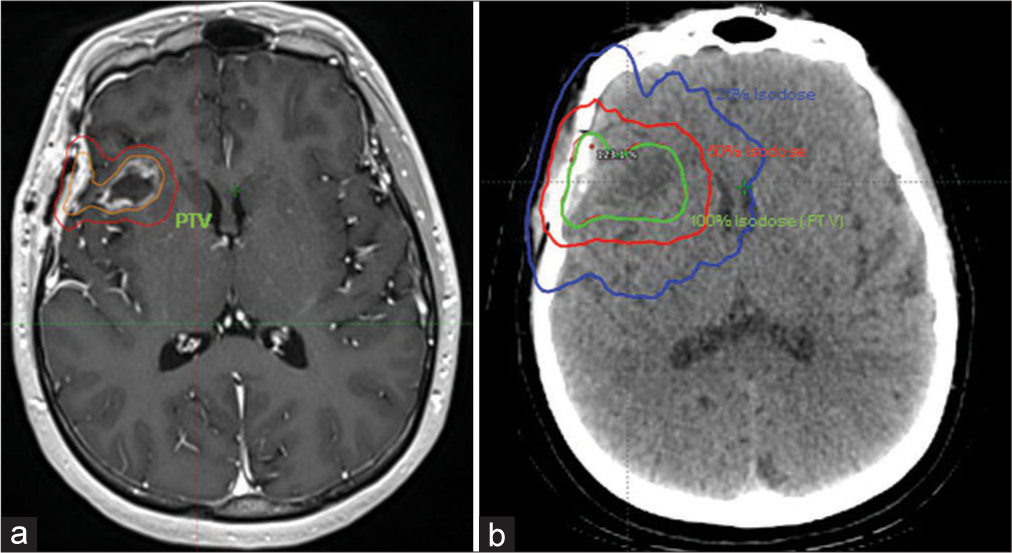
Due to the aforementioned high-risk features, a neurooncology tumor board consensus decision was made to proceed with adjuvant fractionated SRT (FSRT) to the resection cavity. Head CT imaging from the radiation planning session was fused to an MRI T1 post-contrast volumetric sequence for better depiction of the resection cavity. The planning target volume (PTV, volume to where 100% of the dose was prescribed) consisted of the resection cavity plus a 3-mm margin to account for errors during planning and treatment delivery [
Figure 3:
The planning target volume (PTV) (100% of the dose was prescribed to this volume) consisted of the resection cavity (outlined in orange) plus a 3 mm margin (outlined in red) as identified on the T1 post-contrast sequence (a). A 3-arc VMAT plan with 6 megavoltage photons shows the 100% isodose line (21 Gy) covering the PTV target (resection cavity + 3mm margin) on the planning CT head (b).
DISCUSSION
We have highlighted the first account of a primary, malignant, intracranial OFMT, which also represents the first case with extended follow-up to be successfully managed with resection and adjuvant FSRT. Predominately occurring in the lower extremities, OFMTs were historically characterized by their benign pathology and clinical course. Since they were originally believed to have limited potential for local recurrence and distant metastasis, surgical resection followed by surveillance has traditionally been the standard of care. More recently, however, cases of OFMT with malignant pathologic features and a more aggressive clinical course have been increasingly reported.[
In 2003, Folpe and Weiss originally defined pathologic criteria for diagnosing OFMT with malignant behavior.[
While Folpe and others reported that grade and proliferation significantly correlated with rates of local recurrence and distant metastasis among OFMT, other groups have questioned the existence of malignant OFMT. Miettinen et al.[
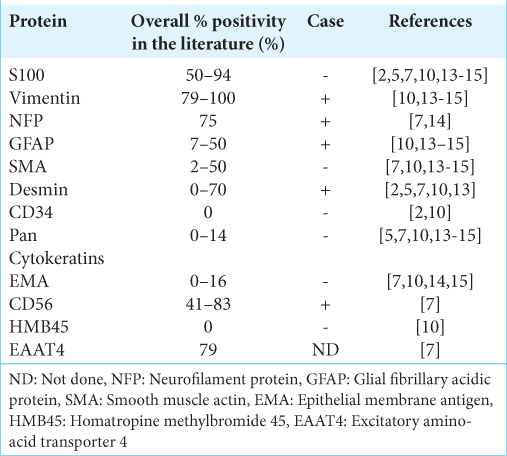
OFMTs closely resemble other mesenchymal tumors under the microscope and therefore immunostaining for molecular markers is crucial for diagnosis. Common immunohistochemical findings of an OFMT are summarized in [
Moreover, while more than two-thirds of OFMTs are positive for S-100 by immunohistochemistry, not all OFMT, including the case discussed in this report, demonstrate S-100 immunostaining [
Gebre-Medhin et al.[
To better understand gene expression differences between subsets of OFMT, Graham et al.[
Another point of contention regarding OFMTs is their lineage of differentiation. While OFMTs were originally thought to be derived from schwannoma or cartilaginous lineages based on published immunohistochemical and ultrastructural analyses,[
To the best of our knowledge, the case discussed in this report, while subject to the limitations inherent to case reports, represents the first OFMT to be diagnosed as a primary case in the CNS. While others have reported paraspinal OFMTs, the paraspinal tumors were believed to have originated in the surrounding muscle and soft tissues with invasion into the spinal canal or bone.[
Historically, the standard of care for OFMTs has been surgical resection with wide margins. Guidelines for adjuvant therapy have not been clearly defined for more aggressive OFMT subtypes and the literature regarding adjuvant radiation and chemotherapy for OFMTs is limited.[
The patient discussed in this report was treated with postoperative SRT due to the high-risk features noted on pathology. Due to the lack of data governing the management of this unique presentation of this rare malignancy, management decisions were extrapolated from studies guiding adjuvant therapy for resected brain metastases. The surgical cavity mildly decreased in size in the 6 weeks between resection and CT simulation yet remained roughly 3 cm in greatest dimension. Historically, to control the rate of radionecrosis, doses for single fraction SRT for lesions of this size are strongly de-escalated. On a recent Phase III trial where doses were progressively de-escalated for increasingly large lesions, 1-year local control was 91% with gross total resection and adjuvant, single fraction SRT for cavities under 2.5 cm in diameter but just 40% for those 2.5–3.5 cm in size.[
In summary, we present the first account of a primary, malignant, intracranial OFMT, which also represents the first case to be successfully managed with resection and adjuvant FSRT. Despite high-risk features consistent with malignant potential as reported in the literature, the patient remains disease and complication free over 5 ½ years after gross total resection and adjuvant FSRT, suggesting that this is a safe and efficacious approach. If OFMTs do indeed demonstrate a neuronal lineage of differentiation, more cases of malignant OFMTs localized to the brain in the future may be encountered. Therefore, it is imperative to identify safe and effective approaches for diagnosing and managing this patient population.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
None.
References
1. Bakiratharajan D, Rekhi B. Ossifying fibromyxoid tumor: An update. Arch Pathol Lab Med. 2016. 140: 371-5
2. Buehler D, Weisman P. Soft tissue tumors of uncertain histogenesis: A review for dermatopathologists. Clin Lab Med. 2017. 37: 647-71
3. Cha JH, Kwon JW, Cho EY, Lee CS, Yoon YC, Choi SH. Ossifying fibromyxoid tumor invading the spine: A case report and review of the literature. Skeletal Radiol. 2008. 37: 1137-40
4. Enzinger FM, Weiss SW, Liang CY. Ossifying fibromyxoid tumor of soft parts. A clinicopathological analysis of 59 cases. Am J Surg Pathol. 1989. 13: 817-27
5. Folpe AL, Weiss SW. Ossifying fibromyxoid tumor of soft parts: A clinicopathologic study of 70 cases with emphasis on atypical and malignant variants. Am J Surg Pathol. 2003. 27: 421-31
6. Gebre-Medhin S, Nord KH, Möller E, Mandahl N, Magnusson L, Nilsson J. Recurrent rearrangement of the PHF1 gene in ossifying fibromyxoid tumors. Am J Pathol. 2012. 181: 1069-77
7. Graham RP, Dry S, Li X, Binder S, Bahrami A, Raimondi SC. Ossifying fibromyxoid tumor of soft parts: A clinicopathologic, proteomic, and genomic study. Am J Surg Pathol. 2011. 35: 1615-25
8. Lehrer EJ, Peterson JL, Zaorsky NG, Brown PD, Sahgal A, Chiang VL. Single versus multifraction stereotactic radiosurgery for large brain metastases: An international meta-analysis of 24 trials. Int J Radiat Oncol Biol Phys. 2019. 103: 618-30
9. Mahajan A, Ahmed S, McAleer MF, Weinberg JS, Li J, Brown P. Post-operative stereotactic radiosurgery versus observation for completely resected brain metastases: A single-centre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017. 18: 1040-8
10. Miettinen M, Finnell V, Fetsch JF. Ossifying fibromyxoid tumor of soft parts-a clinicopathologic and immunohistochemical study of 104 cases with long-term follow-up and a critical review of the literature. Am J Surg Pathol. 2008. 32: 996-1005
11. Park DJJ, Miller NR, Green WR. Ossifying fibromyxoid tumor of the orbit. Ophthalmic Plast Reconstr Surg. 2006. 22: 87-91
12. Schaffler G, Raith J, Ranner G, Weybora W, Jeserschek R. Radiographic appearance of an ossifying fibromyxoid tumor of soft parts. Skeletal Radiol. 1997. 26: 615-8
13. Schofield JB, Krausz T, Stamp GW, Fletcher CD, Fisher C, Azzopardi JG. Ossifying fibromyxoid tumour of soft parts: Immunohistochemical and ultrastructural analysis. Histopathology. 1993. 22: 101-12
14. Williams SB, Ellis GL, Meis JM, Heffner DK. Ossifying fibromyxoid tumour (of soft parts) of the head and neck: A clinicopathological and immunohistochemical study of nine cases. J Laryngol Otol. 1993. 107: 75-80
15. Zámecník M, Michal M, Simpson RH, Lamovec J, Hlavcák P, Kinkor Z. Ossifying fibromyxoid tumor of soft parts: A report of 17 cases with emphasis on unusual histological features. Ann Diagn Pathol. 1997. 1: 73-81