- Department of Neurosurgery, Shariati Hospital, Tehran University of Medical Sciences, Tehran, Iran
- Sport Medicine Research Center, Tehran University of Medical Sciences, Yas Hospital, Tehran, Iran
- Department of Pediatric Neurosurgery, Tehran University of Medical Sciences, Children’s Medical Center, Tehran, Iran
- Department of Neurosurgery, Iran University of Medical Science, Firoozgar Hospital, Tehran, Iran
- Spine Center of Excellence, Yas Hospital, Tehran University of Medical Sciences, Tehran, Iran.
Correspondence Address:
Mohsen Rostami, Spine Center of Excellence, Yas Hospital, Tehran University of Medical Sciences, Tehran, Iran.
DOI:10.25259/SNI_886_2023
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Pedram Jahangiri1, Faramarz Roohollahi2, Zohreh Habibi3, Mohammad Hosein Mirbolouk4, Mohsen Rostami5. Management of aggressive recurrent thoracic spine aneurysmal bone cyst in a 7-year-old male: A case report and review of the literature. 02-Feb-2024;15:30
How to cite this URL: Pedram Jahangiri1, Faramarz Roohollahi2, Zohreh Habibi3, Mohammad Hosein Mirbolouk4, Mohsen Rostami5. Management of aggressive recurrent thoracic spine aneurysmal bone cyst in a 7-year-old male: A case report and review of the literature. 02-Feb-2024;15:30. Available from: https://surgicalneurologyint.com/surgicalint-articles/12731/
Abstract
Background: Spinal aneurysmal bone cysts (ABCs) are rare, histologically benign tumors with aggressive behavior, which may cause bone and soft-tissue destruction, particularly affecting neural elements. Management of these tumors, including treatment modalities and follow-up protocols, remains challenging.
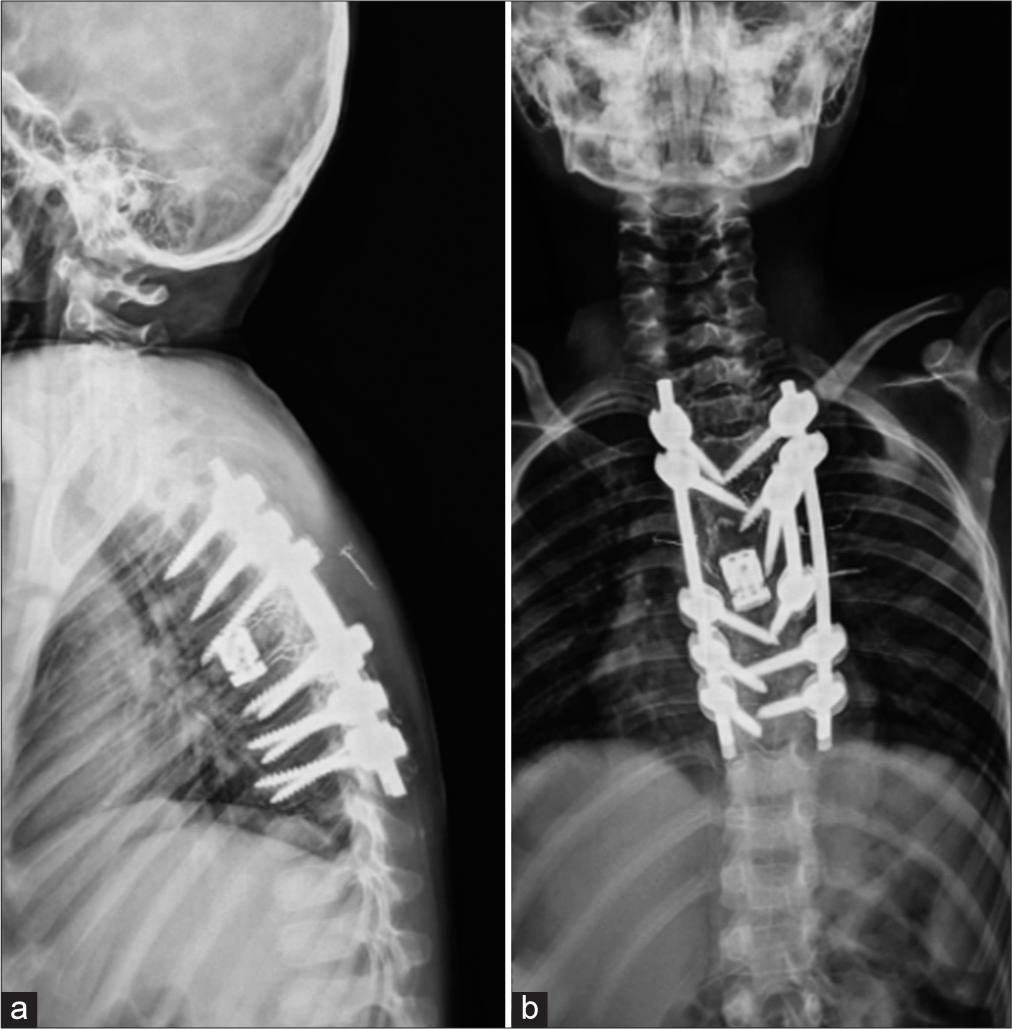
Case Description: A 7-year-old boy presented with chest wall pain persisting for two months before admission, accompanied by progressive mono paresis lasting ten days before admission. Myelopathy signs were evident during the examination. Imaging confirmed a multicystic lesion at the T6 level involving the posterior elements of the vertebra, with significant cord compression. Due to deteriorating neurological function, he underwent urgent laminectomy and neural decompression, followed by subtotal tumor resection. Postoperative histopathological examination confirmed the diagnosis of an ABC, and the patient experienced significant neurological recovery. However, after 21 days, the patient was readmitted to the emergency department with severe paraparesis. Magnetic resonance imaging revealed rapid growth of the residual tumor, leading to cord compression. He underwent aggressive total tumor resection, T6 vertebral body corpectomy, and fixation with pedicle screws and cage insertion. Following the second surgery, prompt neurological recovery occurred.
Conclusion: This rare case report emphasizes the importance of a close follow-up protocol for spinal ABCs in the pediatric population. It highlights the challenges in managing these tumors and the need for vigilant monitoring to detect and address rapid recurrences.
Keywords: Aneurysmal bone cyst, Follow-up, Pediatric, Thoracic spine
INTRODUCTION
Aneurysmal bone cyst (ABC) tumors are rare bone lesions, representing about 1.4% of all primary bone tumors and 15% of all primary spine tumors.[
ABCs are histologically benign; however, due to the possibility of rapid growth and destruction of adjacent bones and soft tissues, they can show aggressive behavior.[
Treatment options for spine ABC tumors include total excision, radiotherapy, and embolization.[
CASE PRESENTATION
A 7-year-old boy with no prior medical history presented at the emergency department with acute left lower extremity monoplegia and sensory loss below the T6 dermatome, which had been ongoing for ten days before admission. Notably, there was no bowel or bladder dysfunction. On physical examination, spasticity in his lower limbs and reduced sensation to pinprick, temperature, and vibration/position below the T6 dermatome were evident. He had been experiencing chest wall pain for the past three months, particularly worsening at night. There was no history of trauma or recent infections reported. All laboratory tests were within normal ranges.
An MRI scan revealed a cystic tumor with internal septations involving the spinous process, lamina, pedicle, and body of the T6 vertebra [
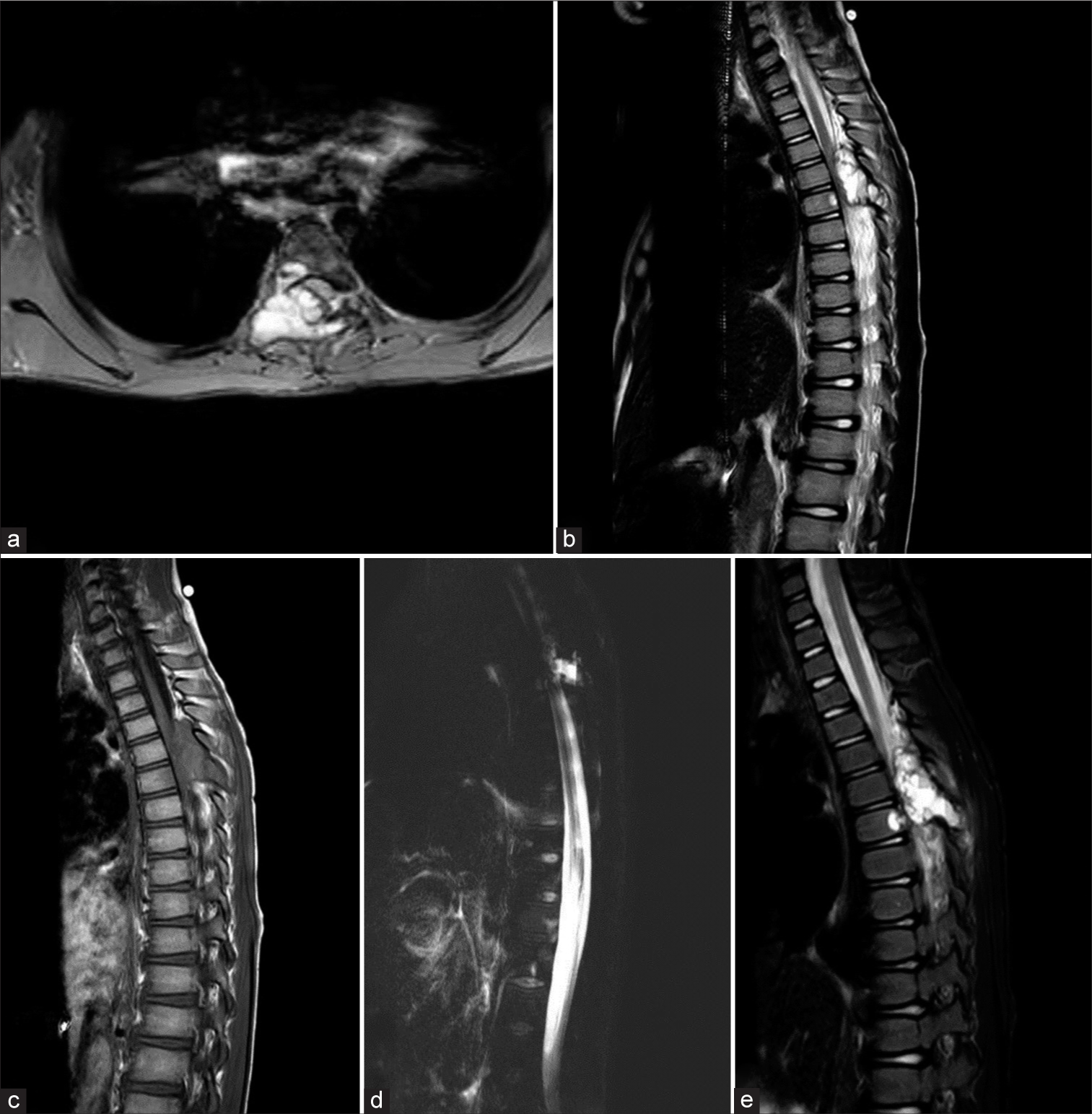
Figure 1:
Preoperative magnetic resonance imaging of the patient: (a) Axial T2 view, (b) sagittal T2 view, (c) sagittal T1 view, (d) myelogram, and (e) short tau inversion recovery (STIR) view showed expansile and heterogeneous cystic bone lesion with fluid signal intensity (T1: Hypointense with T2 and STIR hyperintense) and thin internal septation and cord compression at the T6 vertebra.
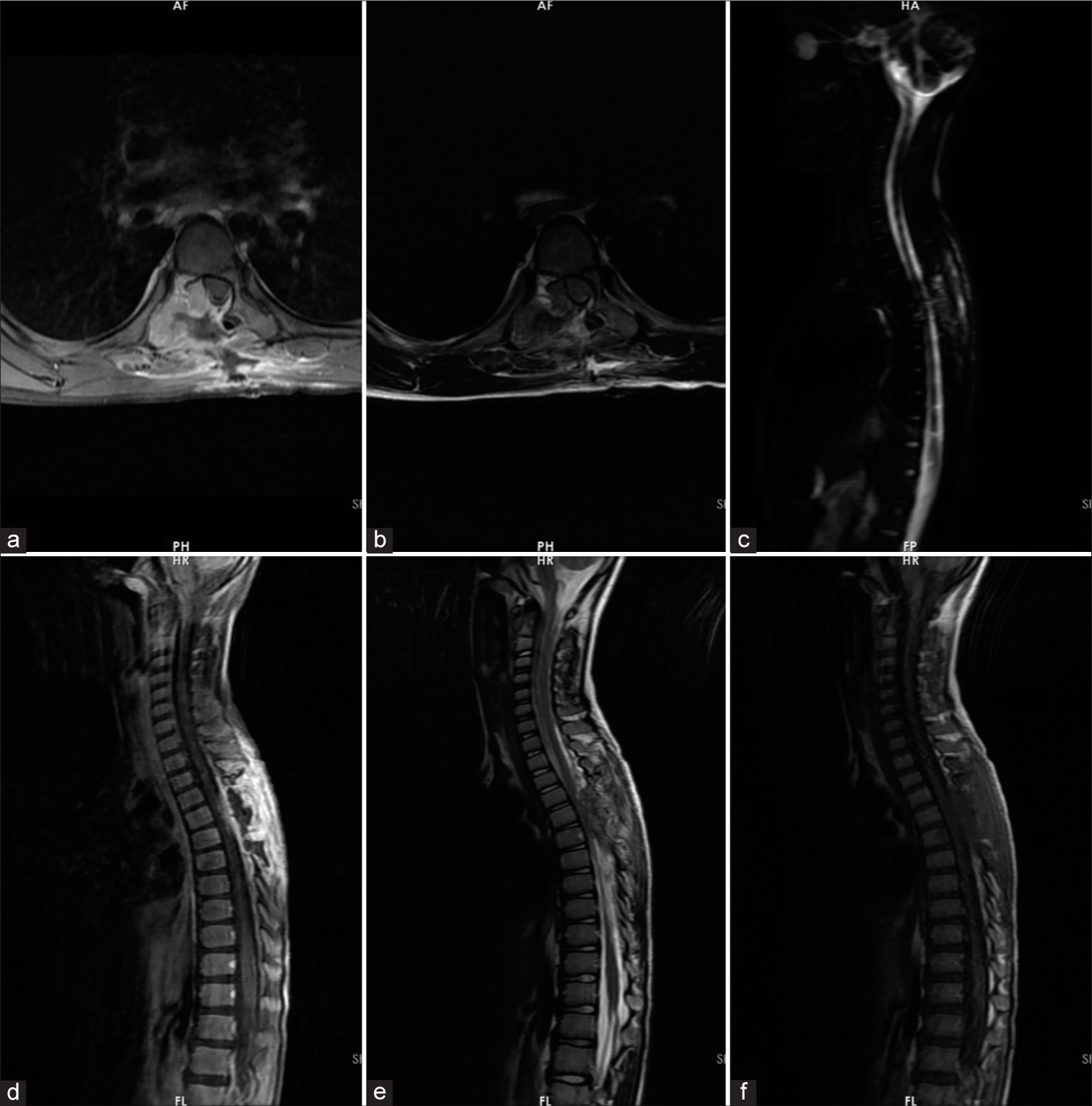
Figure 2:
Follow-up magnetic resonance imaging study of the patient, ten days after the first surgery: (a) T1 with contrast axial view, (b) T2 axial view, (c) myelogram, (d) T1 with contrast sagittal view, (e) T2 sagittal view, and (f) T1 sagittal view showed an expansile and heterogeneous cystic bone lesion with fluid signal intensity (T1: Hypointense with T2 and short tau inversion recovery (STIR) hyperintense) and thin internal septation and cord compression at the T6 vertebra. As shown above, laminectomy and cord decompression were performed. Tumor remnants at the pedicle and posterior parts of the body can be seen. The spinal cord is decompressed relatively.
Twenty-one days after the operation, the patient returned to the emergency department with acute and rapidly worsening severe paraparesis that had started three days prior. The motor strength in both lower extremities was 1/5. MRI revealed rapid tumor growth and significant spinal cord compression [
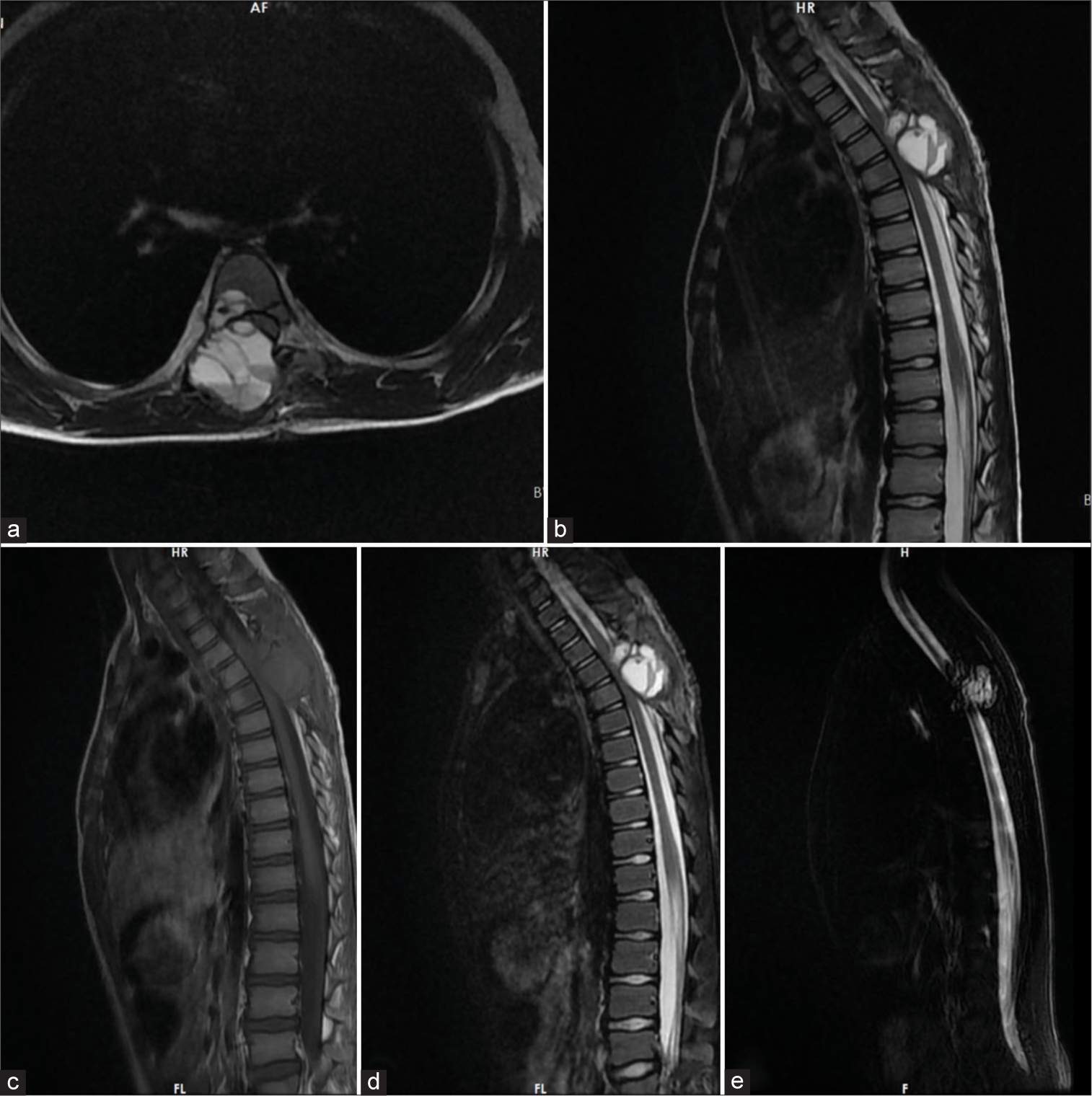
Figure 3:
Magnetic resonance imaging study of the patient before the second surgery (21 days after the first surgery): (a) T2 axial view, (b) T2 sagittal view, (c) T1 sagittal view, (d) sagittal short-tau inversion recovery (STIR) view, and (e) myelogram showed an expansile and heterogeneous cystic bone lesion with fluid signal intensity (T1: Hypointense with T2 and STIR Hyperintense) and thin internal septation and fluid-fluid level and significant cord compression at the T6 vertebra. As seen above, the tumor remnant grew rapidly, leading to cord compression.
DISCUSSION
ABCs are rare, with an annual prevalence of 0.32/100,000 young population.[
The rapid growth of a spinal ABC tumor or vertebral body collapse may cause acute paraplegia due to spinal cord compression.[
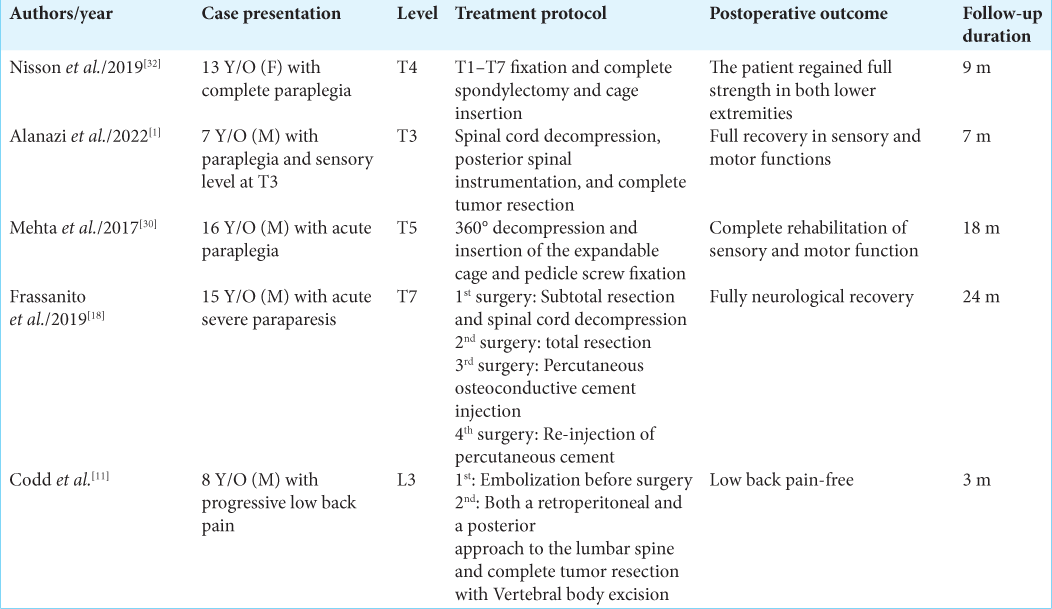
Here, we present the case of a 7-year-old male who exhibited progressive sensorimotor symptoms and experienced significant improvement after the initial spinal cord decompression surgery. While the postoperative MRI study, conducted ten days after the surgery, showed significant cord decompression, the patient was advised to undergo another imaging study 3 months later and return for the next visit. However, the patient returned to the ER with paraparesis 21 days after the initial operation. As shown in
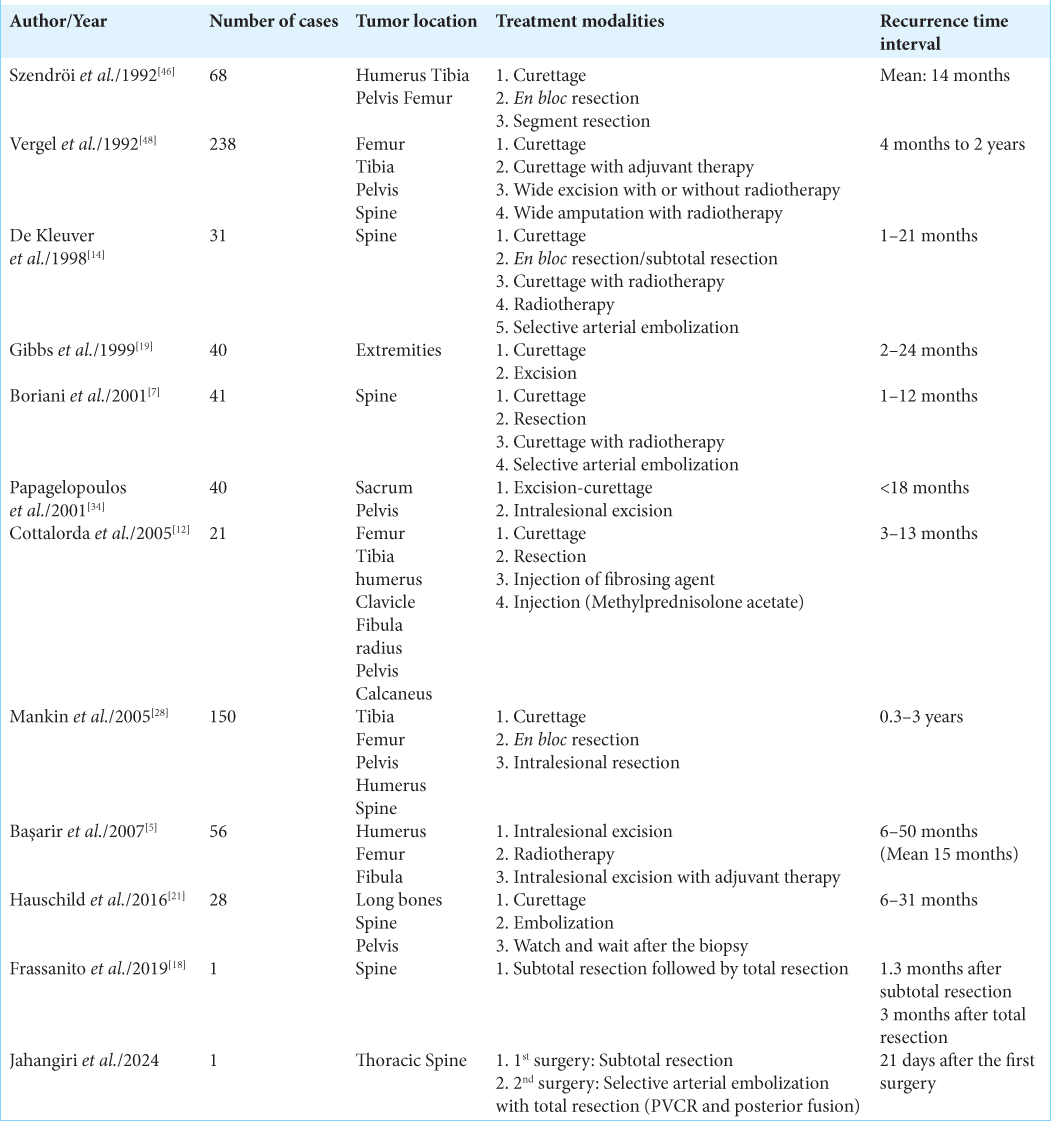
Reviewing the literature, we can find that recurrences have been observed to occur within a range of 1–50 months after treatment. Moreover, as reported by Hauschild et al., 90% of recurrences took place between 6 and 12 months after the initial treatment.[
It is worth noting that in the presented case, the tumor residue in the vertebral body grew rapidly, leading to cord compression and neurological deficits. It is hypothesized that involvement or the presence of anterior parts may be associated with an increased probability of symptoms or a higher rate of recurrence, which is consistent with the previous studies.[
CONCLUSION
ABC tumors, historically considered benign, have the potential to exhibit aggressive behavior. The optimal treatment approach remains uncertain, and the recurrence rate is relatively high. Therefore, close follow-up is strongly recommended. The current follow-up protocol suggests the first visit should occur three months after the initial treatment. However, in cases of subtotal spinal tumor resection, it appears that closer follow-up may be necessary. Furthermore, additional studies are needed to establish a clearer association between vertebral body involvement and the treatment protocol.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Publication of this article was made possible by the James I. and Carolyn R. Ausman Educational Foundation.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript, and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Alanazi OQ, Alshebromi AH, Albaz AF, Bassi MM. Thoracic spine aneurysmal bone cyst causing paraplegia in a child: A case report. J Musculoskelet Surg Res. 2022. 6: 179-83
2. Aljoghaiman MS, Alhamad SM, Homan MA, Harfouch BF. Aneurysmal bone cyst of the spine: Report of four cases and review of the literature. Interdiscip Neurosurg. 2019. 16: 18-21
3. Althof PA, Ohmori K, Zhou M, Bailey JM, Bridge RS, Nelson M. Cytogenetic and molecular cytogenetic findings in 43 aneurysmal bone cysts: Aberrations of 17p mapped to 17p13. 2 by fluorescence in situ hybridization. Mod Pathol. 2004. 17: 518-25
4. Anupam J, Varun V, Prakash K, Rakesh S. Aneurysmal bone cyst of thoracic spine: Case report and brief review of literature. BMJ Case Rep. 2013. 2013: bcr2013009265
5. Başarir K, Pişkin A, Güçlü B, Yildiz Y, Sağlik Y. Aneurysmal bone cyst recurrence in children: A review of 56 patients. J Pediatr Orthop. 2007. 27: 938-43
6. Bollini G, Jouve JL, Cottalorda J, Petit P, Panuel M, Jacquemier M. Aneurysmal bone cyst in children: Analysis of twenty-seven patients. J Pediatr Orthop B. 1998. 7: 274-85
7. Boriani S, De Iure F, Campanacci L, Gasbarrini A, Bandiera S, Biagini R. Aneurysmal bone cyst of the mobile spine: Report on 41 cases. Spine (Phila Pa 1976). 2001. 26: 27-35
8. Campanacci M, Capanna R, Picci P. Unicameral and aneurysmal bone cysts. Clin Orthop Relat Res. 1986. 204: 25-36
9. Chan MS, Wong YC, Yuen MK, Lam D. Spinal aneurysmal bone cyst causing acute cord compression without vertebral collapse: CT and MRI findings. Pediatr Radiol. 2002. 32: 601-4
10. Clough JR, Price CH. Aneurysmal bone cyst: Pathogenesis and long term results of treatment. Clin Orthop Relat Res. 1973. 97: 52-63
11. Codd PJ, Riesenburger RI, Klimo P, Slotkin JR, Smith ER. Vertebra plana due to an aneurysmal bone cyst of the lumbar spine: Case report and review of the literature. J Neurosurg. 2006. 105: 490-5
12. Cottalorda J, Kohler R, Chotel F, de Gauzy JS, Lefort G, Louahem D. Recurrence of aneurysmal bone cysts in young children: A multicentre study. J Pediatr Orthop B. 2005. 14: 212-8
13. Cummings JE, Smith RA, Heck RKJ. Argon beam coagulation as adjuvant treatment after curettage of aneurysmal bone cysts: A preliminary study. Clin Orthop Relat Res. 2010. 468: 231-7
14. De Kleuver M, van der Heul RO, Veraart BE. Aneurysmal bone cyst of the spine: 31 cases and the importance of the surgical approach. J Pediatr Orthop B. 1998. 7: 286-92
15. Desai SB, O’Brien C, Shaikh R, Hedequist D, Proctor M, Orbach DB. Multidisciplinary management of spinal aneurysmal bone cysts: A single-center experience. Interv Neuroradiol. 2019. 25: 564-9
16. Eun J, Oh Y. A case report of aneurysmal bone cyst of the thoracic spine treated by serial anterior and posterior fusion. Medicine (Baltimore). 2019. 98: e17695
17. Feigenberg SJ, Marcus RB, Zlotecki RA, Scarborough MT, Berrey BH, Enneking WF. Megavoltage radiotherapy for aneurysmal bone cysts. Int J Radiat Oncol Biol Phys. 2001. 49: 1243-7
18. Frassanito P, D’Onofrio GF, Pennisi G, Massimi L, Tamburrini G, Muto M. Multimodal management of aggressive recurrent aneurysmal bone cyst of spine: Case report and review of literature. World Neurosurg. 2019. 126: 423-7
19. Gibbs CP, Hefele MC, Peabody TD, Montag AG, Aithal V, Simon MA. Aneurysmal bone cyst of the extremities. Factors related to local recurrence after curettage with a high-speed burr*. JBJS. 1999. 81: 1671-8
20. Harrop JS, Schmidt MH, Boriani S, Shaffrey CI. Aggressive “benign” primary spine neoplasms: Osteoblastoma, aneurysmal bone cyst, and giant cell tumor. Spine. 2009. 34: S39-47
21. Hauschild O, Lüdemann M, Engelhardt M, Baumhoer D, Baumann T, Elger T. Aneurysmal bone cyst (ABC) : Treatment options and proposal of a follow-up regime. Acta Orthop Belg. 2016. 82: 474-83
22. Hay M, Paterson D, Taylor T. Aneurysmal bone cysts of the spine. J Bone Joint Surg Br. 1978. 60: 406-11
23. Keenan S, Bui-Mansfield LT. Musculoskeletal lesions with fluid-fluid level: A pictorial essay. J Comput Assist Tomogr. 2006. 30: 517-24
24. Kieser DC, Mazas S, Cawley DT, Fujishiro T, Tavolaro C, Boissiere L. Bisphosphonate therapy for spinal aneurysmal bone cysts. Eur Spine J. 2018. 27: 851-8
25. Kransdorf MJ, Sweet DE. Aneurysmal bone cyst: Concept, controversy, clinical presentation, and imaging. Am J Roentgenol. 1995. 164: 573-80
26. Leithner A, Windhager R, Lang S, Haas OA, Kainberger F, Kotz R. Aneurysmal bone cyst. A population based epidemiologic study and literature review. Clin Orthop Relat Res. 1999. 363: 176-9
27. Liu JK, Brockmeyer DL, Dailey AT, Schmidt MH. Surgical management of aneurysmal bone cysts of the spine. Neurosurg Focus. 2003. 15: E4
28. Mankin HJ, Hornicek FJ, Ortiz-Cruz E, Villafuerte J, Gebhardt MC. Aneurysmal bone cyst: A review of 150 patients. J Clin Oncol. 2005. 23: 6756-62
29. Mascard E, Gomez-Brouchet A, Lambot K. Bone cysts: Unicameral and aneurysmal bone cyst. Orthop Traumatol Surg Res. 2015. 101: S119-27
30. Mehta V, Padalkar P, Kale M, Kathare A. Solid variant of an aneurysmal bone cyst of the thoracic spine. Cureus. 2017. 9: e1208
31. Nasri E, Reith JD. Aneurysmal bone cyst: A review. J Pathol Transl Med. 2023. 57: 81-7
32. Nisson PL, Link TW, Carnevale J, Virk MS, Greenfield JP. Primary aneurysmal bone cyst of the thoracic spine: A pediatric case report. World Neurosurg. 2020. 134: 408-14
33. Oliveira AM, Chou MM. USP6-induced neoplasms: The biologic spectrum of aneurysmal bone cyst and nodular fasciitis. Hum Pathol. 2014. 45: 1-11
34. Papagelopoulos PJ, Choudhury SN, Frassica FJ, Bond JR, Unni KK, Sim FH. Treatment of aneurysmal bone cysts of the pelvis and sacrum. J Bone Joint Surg Am. 2001. 83: 1674-81
35. Papagelopoulos PJ, Currier BL, Shaughnessy WJ, Sim FH, Ebsersold MJ, Bond JR. Aneurysmal bone cyst of the spine. Management and outcome. Spine (Phila Pa 1976). 1998. 23: 621-8
36. Park HY, Yang SK, Sheppard WL, Hegde V, Zoller SD, Nelson SD. Current management of aneurysmal bone cysts. Curr Rev Musculoskelet Med. 2016. 9: 435-44
37. Parker J, Soltani S, Boissiere L, Obeid I, Gille O, Kieser DC. Spinal aneurysmal bone cysts (ABCs): Optimal management. Orthop Res Rev. 2019. 11: 159-66
38. Pauli C, Fuchs B, Pfirrmann C, Bridge JA, Hofer S, Bode B. Response of an aggressive periosteal aneurysmal bone cyst (ABC) of the radius to denosumab therapy. World J Surg Oncol. 2014. 12: 17
39. Peeters SP, Van der Geest IC, de Rooy JW, Veth RP, Schreuder HW. Aneurysmal bone cyst: The role of cryosurgery as local adjuvant treatment. J Surg Oncol. 2009. 100: 719-24
40. Rahman MA, El Masry AM, Azmy SI. Review of 16 cases of aneurysmal bone cyst in the proximal femur treated by extended curettage and cryosurgery with reconstruction using autogenous nonvascularized fibula graft. J Orthop Surg. 2018. 26: 2309499018783905
41. Restrepo R, Zahrah D, Pelaez L, Temple HT, Murakami JW. Update on aneurysmal bone cyst: Pathophysiology, histology, imaging and treatment. Pediatr Radiol. 2022. 52: 1601-14
42. Rossi G, Rimondi E, Bartalena T, Gerardi A, Alberghini M, Staals EL. Selective arterial embolization of 36 aneurysmal bone cysts of the skeleton with N-2-butyl cyanoacrylate. Skeletal Radiol. 2010. 39: 161-7
43. Sayago LR, Remondino RG, Tello CA, Piantoni L, Wilson IA, Galaretto E. Aneurysmal bone cysts of the spine in children: A review of 18 cases. Global Spine J. 2019. 10: 875-80
44. Shiels WE, Mayerson JL. Percutaneous doxycycline treatment of aneurysmal bone cysts with low recurrence rate: A preliminary report. Clin Orthop Relat Res. 2013. 471: 2675-83
45. Steffner RJ, Liao C, Stacy G, Atanda AJ, Attar S, Avedian R. Factors associated with recurrence of primary aneurysmal bone cysts: Is argon beam coagulation an effective adjuvant treatment?. J Bone Joint Surg Am. 2011. 93: e1221-9
46. Szendröi M, Cser I, Kónya A, Rényi-Vámos A. Aneurysmal bone cyst. A review of 52 primary and 16 secondary cases. Arch Orthop Trauma Surg. 1992. 111: 318-22
47. Tsagozis P, Brosjö O. Current strategies for the treatment of aneurysmal bone cysts. Orthop Rev (Pavia). 2015. 7: 6182
48. Vergel De Dios AM, Bond JR, Shives TC, McLeod RA, Unni KK. Aneurysmal bone cyst. A clinicopathologic study of 238 cases. Cancer. 1992. 69: 2921-31
49. Ye Y, Pringle LM, Lau AW, Riquelme DN, Wang H, Jiang T. TRE17/USP6 oncogene translocated in aneurysmal bone cyst induces matrix metalloproteinase production via activation of NF-κB. Oncogene. 2010. 29: 3619-29
50. Zehetgruber H, Bittner B, Gruber D, Krepler P, Trieb K, Kotz R. Prevalence of aneurysmal and solitary bone cysts in young patients. Clin Orthop Relat Res. 2005. 439: 136-43