- Department of Neurosurgery, The University of Texas Health San Antonio, San Antonio, Texas, United States
- Department of Neurosurgery, Medical College of Wisconsin, Milwaukee, Wisconsin, United States
- Department of Otolaryngology-Head and Neck Surgery, The University of Texas Health San Antonio, San Antonio, Texas, United States
Correspondence Address:
Jonathan Espinosa, Department of Neurosurgery, The University of Texas Health San Antonio, San Antonio, Texas, United States.
DOI:10.25259/SNI_294_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Jonathan Espinosa1, Samon Tavakoli2, Philip Chen3, Justin Mascitelli1, Cristian Gragnaniello1. Management of concurrent symptomatic tuberculum sellae meningioma and idiopathic intracranial hypertension: A case report. 23-Aug-2024;15:298
How to cite this URL: Jonathan Espinosa1, Samon Tavakoli2, Philip Chen3, Justin Mascitelli1, Cristian Gragnaniello1. Management of concurrent symptomatic tuberculum sellae meningioma and idiopathic intracranial hypertension: A case report. 23-Aug-2024;15:298. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13056
Abstract
Background: Coexisting intracranial pathologies of distinct etiology which require intervention are rare. Only a handful of cases have been reported in the literature. The effects of each treatment option on both pathologies need to be considered during management. We describe the first report of the management of a patient with concurrent symptomatic tuberculum sellae meningioma (TSM) and idiopathic intracranial hypertension (IIH).
Case Description: A 58-year-old male presented with 2 weeks of vision loss and 3 months of headaches. He was found to have an inferior hemi-field deficit in the left eye and bilateral papilledema. Imaging studies revealed bilateral transverse sinus stenosis and a TSM abutting the left optic nerve. The opening pressure was 40 cmH2O. An expanded-endoscopic endonasal approach was performed for mass resection. Intraoperatively, a lumbar drain was placed to aid skull base repair integrity before definitive treatment was obtained. On postoperative day 9, a right transverse-sigmoid sinus stent was placed for IIH treatment. The patient was discharged the following day.
Conclusion: Our management of this patient targeted the etiologies of each symptomatic pathology. Stenting provided treatment for the IIH and mass resection for the vision loss. Both the order and approaches to treatment were felt to maximize patient benefit while minimizing harm.
Keywords: Case report, Idiopathic intracranial hypertension, Symptomatic, Tuberculum sellae meningioma
INTRODUCTION
Careful consideration of all treatment options available is essential in the management of complex cases. We recently treated a patient with concurrent tuberculum sellae meningioma (TSM) and idiopathic intracranial hypertension (IIH) who presented with headaches, papilledema, and focal visual defect. We describe the rationale and considerations for the management of this patient.
IIH is a diagnosis of exclusion with an unclear etiology.[
TSMs are a subset of typically benign intracranial tumors that arise from the meningeal layer of the tuberculum sellae. Depending on the size and growth pattern of the tumor, patients may be asymptomatic and have headaches or visual field deficits.[
While both entities have a unique pathophysiology, their presenting symptoms can overlap, which complicates the development of an optimal therapeutic plan. Concurrent intracranial pathologies of distinct etiology requiring surgical intervention are rare, with only a handful of cases reported in the literature.[
CASE DESCRIPTION
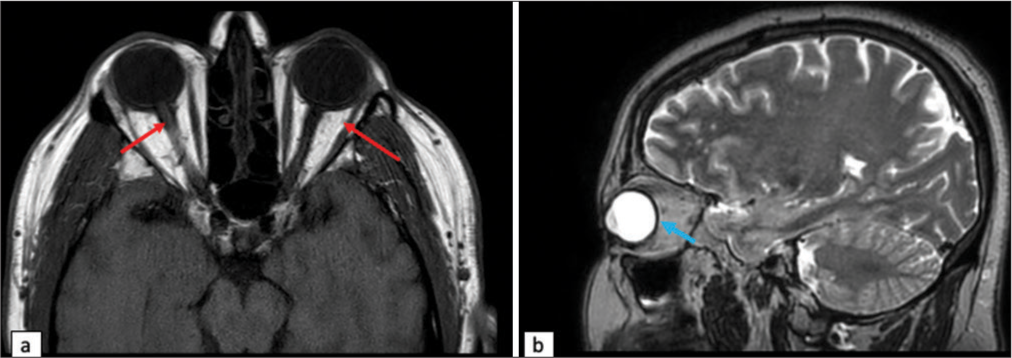
We present a 58-year-old male with class I obesity (body mass index [BMI] 31.0), a history of renal cell carcinoma in remission status post gross total resection, hypertension, and diabetes mellitus type 2, evaluated as an outpatient by an ophthalmologist for a 2-week history of vision loss in his left eye and 3 months of mild frontal headaches. He was found to have inferior nasal and temporal field defects in his left eye with bilateral papilledema. His visual acuity (VA) was 20/25 on the right and 20/30 on the left. He was sent to the emergency department for further work up. Initial head computed tomography (CT) was suspicious for bilateral transverse sinus stenosis (TSS) and a mass in the suprasellar region. A CT venogram was obtained and confirmed bilateral TSS. Magnetic resonance imaging (MRI) confirmed the presence of a homogeneously enhancing, dural-based tuberculum sellae mass with involvement of the left optic nerve and proximal segment of the left anterior cerebral artery [
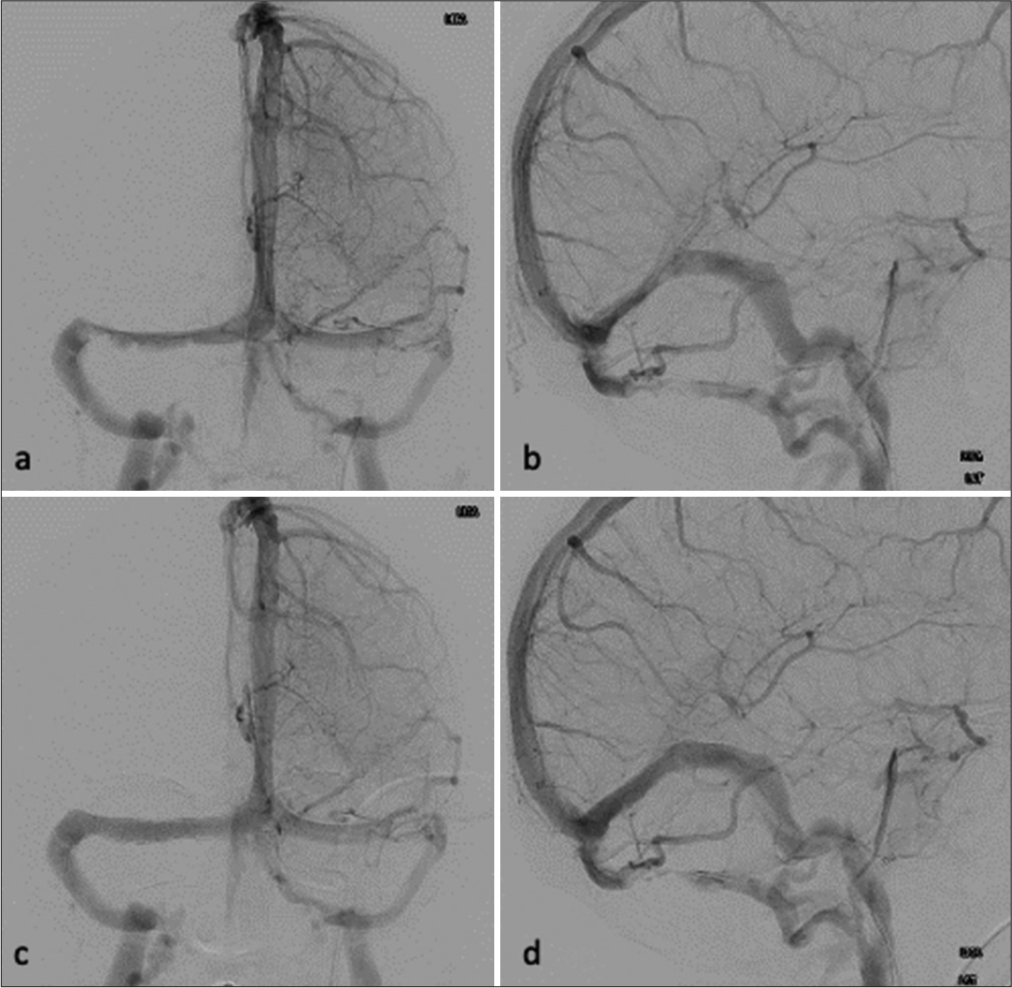
The patient underwent an expanded endoscopic endonasal approach for surgical resection of the mass. Intraoperatively, a lumbar drain was placed. Skull base repair was done, implementing both an inlay and overlay graft to create a dural substitute. A pedicle of the nasal septal flap was kept intact to maintain its blood supply. The vascularized flap was placed on top of the overlay and covered with surgical sealant. Postoperatively, the patient reported a slight improvement in vision. The lumbar drain was removed on postoperative day 7. On postoperative day 8, the patient was loaded with 650 mg of aspirin and 300 mg of clopidogrel. On postoperative day 9, the patient was taken to the interventional radiology suite to undergo VSS. The angiogram confirmed bilateral TSS, worse on the left than the right; however, the right side appeared to be the dominant sinus. A right transverse-sigmoid sinus stent was subsequently placed with the pressure gradient decreasing from 11 mm Hg to 3 mm Hg. There was no evidence of complications [
Figure 3:
Preoperative (a) left anteroposterior (AP) internal carotid artery (ICA) and (b) left lateral ICA injections demonstrating bilateral transverse sinus stenosis. Postoperative (c) left AP ICA and (d) left lateral ICA injections demonstrating improved stenosis of the right transverse sinus after venous sinus stent placement.
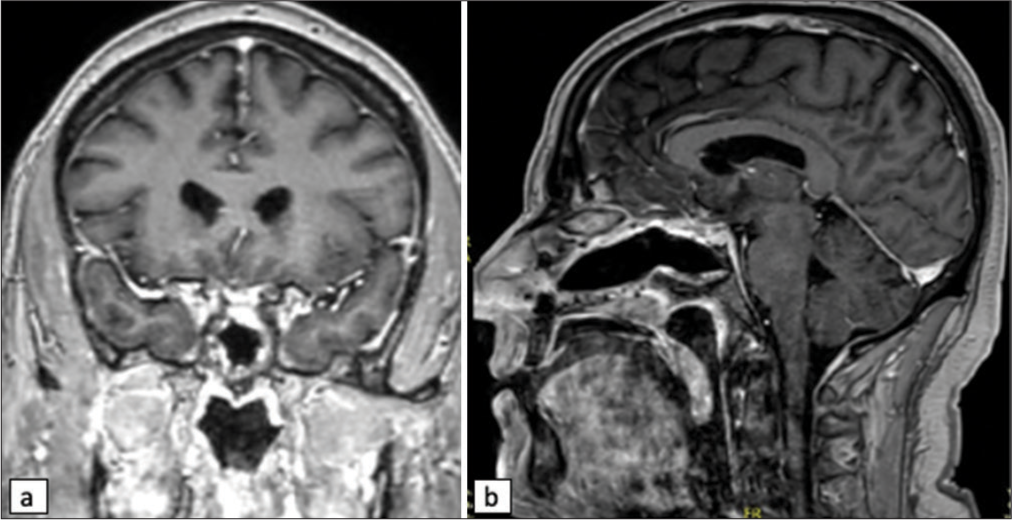
Pathology was consistent with a World Health Organization grade 1 meningioma; therefore, no plans were made for postoperative radiation. He has been followed with serial imaging. Radiographic outcomes have demonstrated gross total resection of the TSM without evidence of recurrence and patency of the right sinus stent [
DISCUSSION
The management of patients with concurrent intracranial pathologies presents unique challenges to developing an optimal treatment plan. Careful consideration must be given to both while prioritizing the treatment to address the patient’s symptoms.[
The treatment for IIH was not thought to address the visual field cut, and undergoing a venous stent procedure would require the patient to be on dual anti-platelet therapy (DAPT). This would have delayed a possible surgical decompression of the left optic nerve for at least 3–6 months after stent placement, during which time there would have been a high concern for deterioration of the patient’s vision. In the case of deterioration, the patient would have faced worsening vision with no intervention or would have risked in-stent thrombosis secondary to the cessation of DAPT to perform surgery. Contrarily, it was felt that VSS with DAPT therapy could safely be initiated within a week after surgical resection, avoiding the risks mentioned above.
Many factors were considered when choosing the optimal surgical approach. An endonasal approach was indicated here since the tumor was small and located between the carotid arteries and below the optic apparatus.[
CONCLUSION
The treatment plan for this patient was complex due to the presence of two distinct pathologies potentially contributing to overlapping symptoms. The diagnostic workup for these two pathologies and treatment options available when these illnesses occur simultaneously is no different than when they occur in isolation. A sophisticated treatment plan was necessary to address both pathologies individually, as the management of one pathology complicated that of the other and vice versa. Most importantly, the finalized treatment plan should minimize harm to the patient.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Bander ED, Singh H, Ogilvie CB, Cusic RC, Pisapia DJ, Tsiouris AJ. Endoscopic endonasal versus transcranial approach to tuberculum sellae and planum sphenoidale meningiomas in a similar cohort of patients. J Neurosurg. 2018. 128: 40-8
2. Bowers CA, Altay T, Couldwell WT. Surgical decision-making strategies in tuberculum sellae meningioma resection. Neurosurg Focus. 2011. 30: E1
3. Clark AJ, Jahangiri A, Garcia RM, George JR, Sughrue ME, McDermott MW. Endoscopic surgery for tuberculum sellae meningiomas: A systematic review and meta-analysis. Neurosurg Rev. 2013. 36: 349-59
4. Cohen S, Jones SH, Dhandapani S, Negm HM, Anand VK, Schwartz TH. Lumbar drains decrease the risk of postoperative cerebrospinal fluid leak following endonasal endoscopic surgery for suprasellar meningiomas in patients with high body mass index. Oper Neurosurg (Hagerstown). 2018. 14: 66-71
5. Dlouhy BJ, Madhavan K, Clinger JD, Reddy A, Dawson JD, O’Brien EK. Elevated body mass index and risk of postoperative CSF leak following transsphenoidal surgery. J Neurosurg. 2012. 116: 1311-7
6. Elshazly K, Kshettry VR, Farrell CJ, Nyquist G, Rosen M, Evans JJ. Clinical outcome after endoscopic endonasal resection of tuberculum sella meningiomas. Oper Neurosurg (Hagerstown). 2018. 14: 494-502
7. Fargen KM, Liu K, Garner RM, Greeneway GP, Wolfe SQ, Crowley RW. Recommendations for the selection and treatment of patients with idiopathic intracranial hypertension for venous sinus stenting. J Neurointerv Surg. 2018. 10: 1203-8
8. Fraser S, Gardner PA, Koutourousiou M, Kubik M, Fernandez-Miranda JC, Snyderman CH. Risk factors associated with postoperative cerebrospinal fluid leak after endoscopic endonasal skull base surgery. J Neurosurg. 2018. 128: 1066-71
9. Friedman DI, Liu GT, Digre KB. Revised diagnostic criteria for the pseudotumor cerebri syndrome in adults and children. Neurology. 2013. 81: 1159-65
10. Greener DL, Akarca D, Durnford AJ, Ewbank F, Buckland GR, Hempenstall J. Idiopathic intracranial hypertension: Shunt failure and the role of obesity. World Neurosurg. 2020. 137: e83-8
11. Karsy M, Raheja A, Eli I, Guan J, Couldwell WT. Clinical outcomes with transcranial resection of the tuberculum sellae meningioma. World Neurosurg. 2017. 108: 748-55
12. Kinali B, Senoglu M, Sandal E, Sandhu G, Karadag A. Management strategy for a patient with coexistence of meningioma and paraophthalmic aneurysm. J Coll Physicians Surg Pak. 2021. 30: 585-7
13. Leishangthem L, SirDeshpande P, Dua D, Satti SR. Dural venous sinus stenting for idiopathic intracranial hypertension: An updated review. J Neuroradiol. 2019. 46: 148-54
14. Liu X, Di H, Wang J, Cao X, Du Z, Zhang R. Endovascular stenting for idiopathic intracranial hypertension with venous sinus stenosis. Brain Behav. 2019. 9: e01279
15. Magill ST, McDermott MW. Tuberculum sellae meningiomas. Handb Clin Neurol. 2020. 170: 13-23
16. Michael AP, Elbuluk O, Tsiouris AJ, Tabaee A, Kacker A, Anand VK. The critical importance of a vascularized flap in preventing recurrence after endoscopic repair of spontaneous cerebrospinal fluid leaks and meningoencephaloceles. J Neurosurg. 2021. 137: 79-86
17. Nia AM, Srinivasan VM, Lall R, Kan P. Dural venous sinus stenting in idiopathic intracranial hypertension: A national database study of 541 patients. World Neurosurg. 2022. 167: e451-5
18. Satti SR, Leishangthem L, Chaudry MI. Meta-analysis of CSF diversion procedures and dural venous sinus stenting in the setting of medically refractory idiopathic intracranial hypertension. AJNR Am J Neuroradiol. 2015. 36: 1899-904
19. Takeda N, Nishihara M, Yamanishi S, Kidoguchi K, Hashimoto K. Strategy for patients with co-existence of meningioma and intracerebral aneurysm, especially unruptured aneurysm (-seven cases and review of the literature-). J Clin Neurosci. 2017. 45: 236-42
20. Tanioka S, Fujiwara M, Yago T, Tanaka K, Ishida F, Suzuki H. Glioblastoma with concomitant moyamoya vasculopathy in neurofibromatosis type 1: Illustrative case. J Neurosurg Case Lessons. 2022. 3: CASE21708
21. Teachey W, Grayson J, Cho DY, Riley KO, Woodworth BA. Intervention for elevated intracranial pressure improves success rate after repair of spontaneous cerebrospinal fluid leaks. Laryngoscope. 2017. 127: 2011-6
22. Toscano S, Lo Fermo S, Reggio E, Chisari CG, Patti F, Zappia M. An update on idiopathic intracranial hypertension in adults: A look at pathophysiology, diagnostic approach and management. J Neurol. 2021. 268: 3249-68
23. Wang MT, Bhatti MT, Danesh-Meyer HV. Idiopathic intracranial hypertension: Pathophysiology, diagnosis and management. J Clin Neurosci. 2022. 95: 172-9
24. Zocchi J, Pietrobon G, Lepera D, Gallo S, Russo F, Volpi L. Spontaneous CSF leaks and IIH: A flawless connection? An experience with 167 patients. Laryngoscope. 2021. 131: E401-7
25. Zwagerman NT, Wang EW, Shin SS, Chang YF, Fernandez-Miranda JC, Snyderman CH. Does lumbar drainage reduce postoperative cerebrospinal fluid leak after endoscopic endonasal skull base surgery? A prospective, randomized controlled trial. J Neurosurg. 2018. 131: 1172-8