- College of Medicine, King Saud bin Abdulaziz University for Health Sciences, Jeddah, Saudi Arabia.
- King Abdullah International Medical Research Center, Jeddah, Saudi Arabia.
- Department of Neurosciences, King Faisal Specialist Hospital and Research Center, Jeddah, Saudi Arabia.
Correspondence Address:
Abdulaziz Hamzah, College of Medicine, King Saud bin Abdulaziz University for Health Sciences, Jeddah, Saudi Arabia.
DOI:10.25259/SNI_441_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Abdulaziz Hamzah1,2, Abdullah S. Alharbi1,2, Ahmed S. Abdulhamid1,2, Alaa Nabil Turkistani3, Mohmmed Hani Aref3. Management of postoperative cerebral vasospasm in skull base surgeries: A systematic review of case reports and series. 23-Jun-2023;14:214
How to cite this URL: Abdulaziz Hamzah1,2, Abdullah S. Alharbi1,2, Ahmed S. Abdulhamid1,2, Alaa Nabil Turkistani3, Mohmmed Hani Aref3. Management of postoperative cerebral vasospasm in skull base surgeries: A systematic review of case reports and series. 23-Jun-2023;14:214. Available from: https://surgicalneurologyint.com/surgicalint-articles/management-of-postoperative-cerebral-vasospasm-in-skull-base-surgeries-a-systematic-review-of-case-reports-and-series/
Abstract
Background: This study provides a comprehensive overview of the management of postoperative vasospasm after skull base surgeries. This phenomenon is rare but can be of serious sequelae.
Methods: Medline, Embase, and PubMed Central were searched, along with examining the references of the included studies. Only case reports and series that reported vasospasm following a skull base pathology were incorporated. Cases with pathologies other than skull base, subarachnoid hemorrhage, aneurysm, and reversible cerebral vasoconstriction syndrome were excluded from the study. Quantitative data were presented as mean (Standard Deviation) or median (range), accordingly, while qualitative data were presented as frequency (percentage). Chi- square test and one-way analysis of variance were used to assess for any association between the different factors and patient outcomes.
Results: We had a total of 42 cases extracted from the literature. The mean age was 40.1 (±16.1) with approximately equal males and females (19 [45.2%] and 23 [54.8%], respectively). The time to develop vasospasm after the surgery was 7 days (±3.7). Most of the cases were diagnosed by either angiogram or magnetic resonance angiography. Seventeen of the 42 patients had pituitary adenoma as the pathology. Anterior circulation was nearly affected in all patients. For management, most patients received pharmacological with supportive management. Twenty-three patients had an incomplete recovery as a result of vasospasm.
Conclusion: Vasospasm following skull base operations can affect males and females, and most patients in this review were middle-aged adults. The outcome of patients varies; however, most patients did not achieve a full recovery. There was no correlation between any factors and the outcome.
Keywords: Cerebral vasospasm, Skull base surgery, Skull base tumors, Systematic review, Vasospasm management, Vasospasm
INTRODUCTION
Although cerebral vasospasm is a known complication of subarachnoid hemorrhage (SAH), there have been several reports in which vasospasm has occurred following tumor resection.[
The present study aims to provide a comprehensive overview of the management of postoperative vasospasm after skull base surgeries alongside the patients’ demographic data, diagnostic methods, and the outcomes of the surgeries. Statistical analysis is conducted to determine which factors are attributed to the complete recovery.
MATERIALS AND METHODS
In this systematic review, Medline, Embase, and PubMed Central (PMC) were searched without any limitations on the date of publication. The keywords used in combinations were as follows: cerebral vasospasm, cranial vasospasm, cerebrovasospasm, brain vasospasm, postoperative, postsurgery, postoperative complication, postoperative vasospasm, removal, and resection. The included papers were supplemented by examining the references of the papers. Case reports and series that reported cerebral vasospasm following skull base surgeries were included in the study, while reports of cases other than skull base pathologies, cases with SAH, aneurysms, and reversible cerebral vasoconstriction syndrome were excluded from the study. The search was conducted in accordance with a predetermined protocol and reported in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analysis. Quantitative data were presented as the mean and standard deviation or median and range, while qualitative data were presented as frequency and percentage. Chi-square test and one-way analysis of variance (ANOVA) were used to analyze for any association between the examined factors and patient outcome.
Eligibility and selection criteria
Case reports and series of skull base surgeries associated with postoperative vasospasm were the only studies included in the study, while studies that included SAH, aneurysm, and reversible cerebral vasospasm syndrome were excluded from the study. Titles and abstracts that met our prespecified criteria were reviewed by two independent investigators and in duplicate. After that, the same investigators assessed the full-text of the included studies for eligibility. In addition, the relevant information was extracted from the studies that met our inclusion criteria in a prespecified data collection sheet. A third investigator would be needed to resolve any disagreement if it was to occur.
Data synthesis and analysis
The data extracted from the included articles (author, year of publication, country, and journal), study design (case report and case series), demographics (age of subjects and sex), clinical features (presentation and time to develop vasospasm), disease features (pathology, location, and which circulation affected), diagnostics techniques (imaging such as angiogram, magnetic resonance angiography [MRA], or computed tomography angiography [CTA]), type of surgery, type of management (pharmacology, intervention, or supportive), the outcome (whether it was complete [patient neurological status has returned to its baseline immediate postoperative status], incomplete recovery [no or gradual improvement in deficit] or death), follow-up, and the outcome of the follow-up. Studies were screened based on the inclusion and exclusion criteria that were mentioned above by one group of two authors independently.
The senior author was involved in the process of reviewing and discussing the variations in the study selection and the quality of the papers. SPSS (Release 23.0.0.0, IBM, USA) was used in the process of data management as well as the analysis. Descriptive statistics were used in the formulation of a summary of baseline demographics and clinical characteristics. Categorical variables will be expressed in the form of frequency and percentages, while continuous variables will be expressed as mean and standard deviation, or median and interquartile range if not normally distributed. To determine the association between the patients characteristics and the outcomes of the postoperative vasospasm, ANOVA and Chi- square tests were used accordingly.
RESULTS
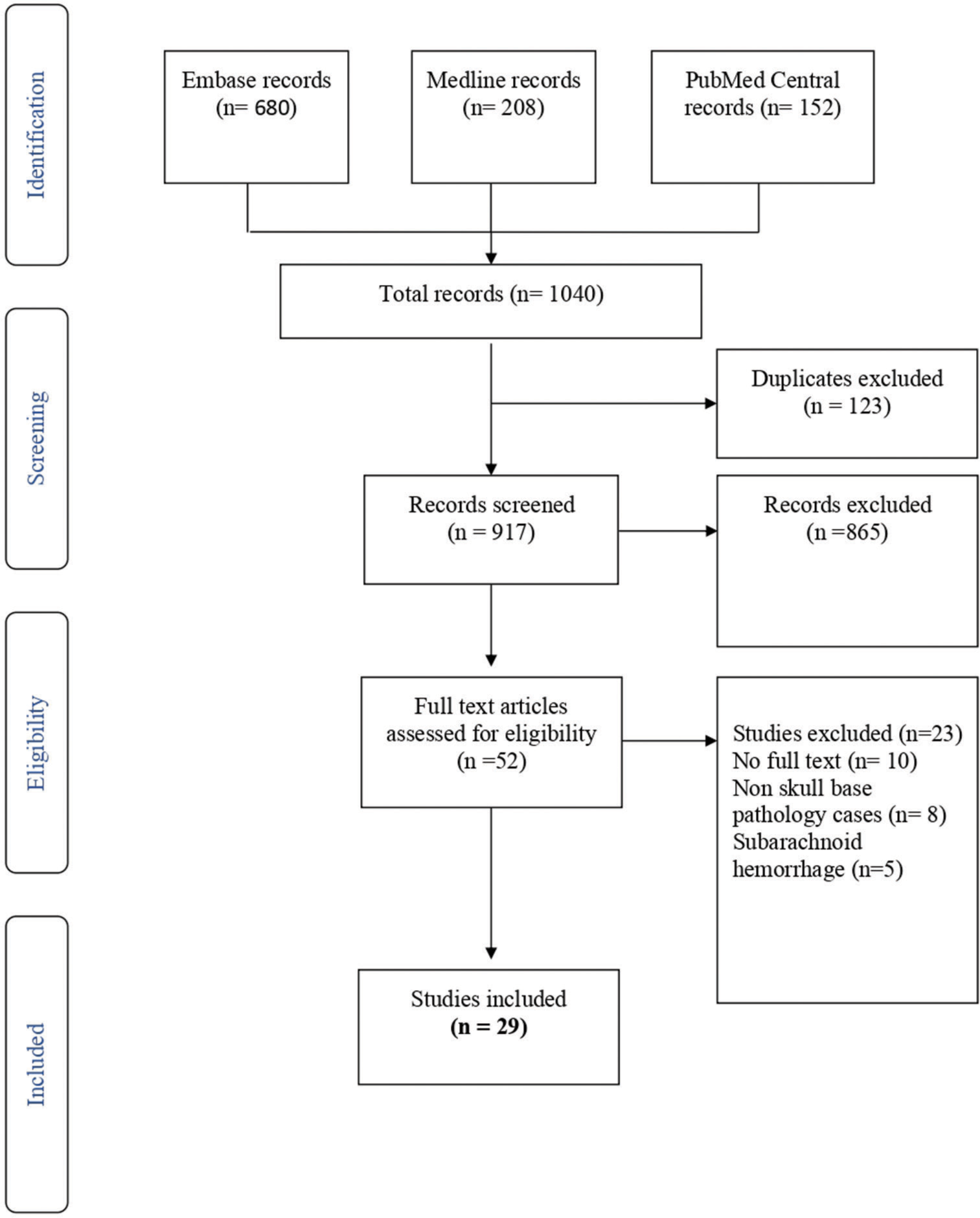
Embase, Medline, and PMC records generated 680, 208, and 152 articles, respectively. In the total of 1040 articles, 123 duplicates were excluded from the study. Title and abstract screening excluded 865 articles. Fifty-two papers were potentially relevant and underwent full-text screening, which resulted in the exclusion of 23 papers for lack of full text (10 papers), pathologies other than skull base (8), and SAH (5) [
The net relevant cases were 29, supplemented by reference screening relevant articles, and 11 were added manually. Twenty-nine articles reporting 42 cases are included, analyzed, and summarized in
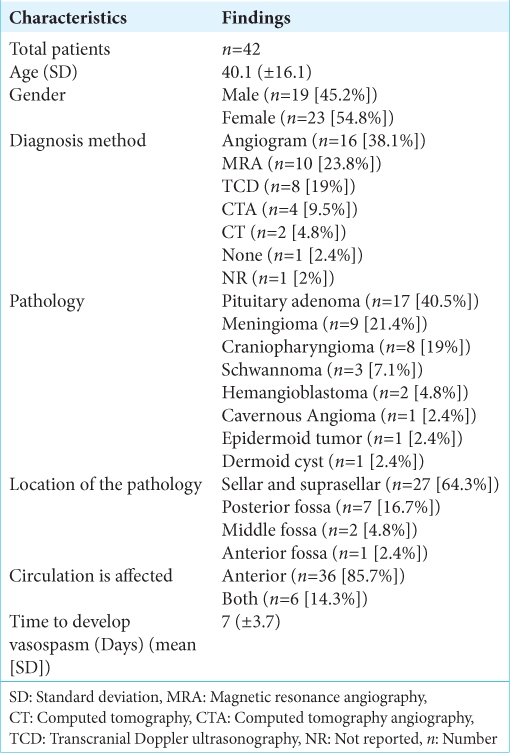
Presentation and diagnosis
In terms of demographics, females comprised 23 cases versus 19 males. The mean age was 40.1 (±16.1). Regarding clinical presentation, visual disturbance was the most significant symptom reported in 42% of cases. Hemiparesis and mental status changes were reported in three each. The majority of cerebral vasospasm cases were diagnosed using cerebral angiography (38.1% of cases) followed by MRA in 23.8%.
Operative data
The performed operations were either endoscopic or open surgeries. Open surgeries with different approaches were reported in 24 cases (57.1%), while endoscopic surgeries comprised 16 cases (38.1%). There was one case of combined endoscopic and open surgery. The most common location of lesions was sellar/suprasellar space in 64.3% of cases, far from the following location, the posterior fossa, which was reported in 16.7% of cases. The anterior cranial fossa was the least reported location associated with postoperative vasospasm. On the other hand, the most common pathology was pituitary adenoma, comprising of 17 (40.5%) cases followed by meningioma in 9 (21.4%) cases. Craniopharyngioma, schwannoma, and hemangioblastoma comprised 8, 3, and 2 cases, respectively.
Circulation and vasospasm time
Almost all patients had the anterior circulation affected, whether alone (85.7%) or combined (14.3%). The average time to develop vasospasm following surgeries was 7 days (±3.7). The earliest time to develop vasospasm was one day following a case of hemangioblastoma and a case of sphenoclinoidocavernous meningioma. On the other hand, the latest period was 14 days after the epidermoid tumor and craniopharyngioma resection.
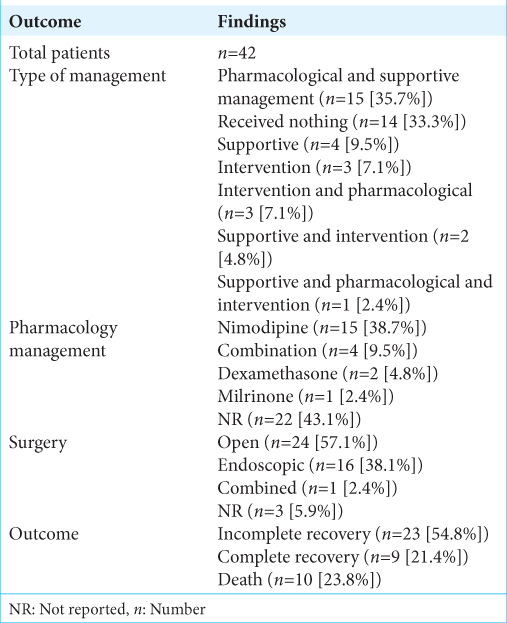
Management and outcome
In terms of management, data are summarized in
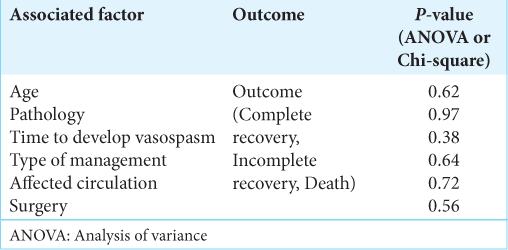
Inferential
Cross-tabulation between the outcome and the management strategy and statistical analysis showed no significant correlation between the outcome and whether the patient was managed with pharmacological and supportive, pharmacological and interventional, supportive and interventional, supportive only, interventional only, or no management at all [
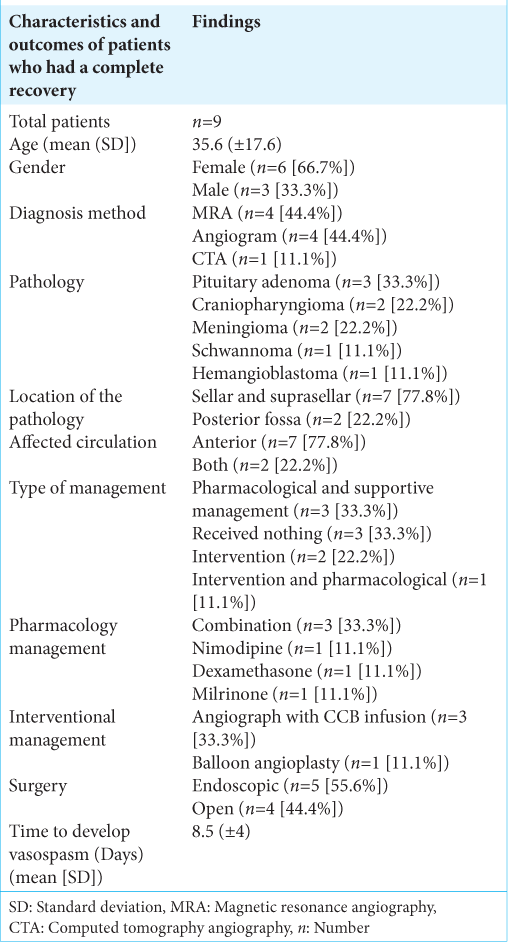
Patients who had a complete recovery
A total of nine patients had a complete recovery, and their data are summarized in
DISCUSSION
Brain tumor surgeries can lead to several potential complications, one of which is cerebral vasospasm.[
Cerebral vasospasm is a well-known complication following SAH, but it can also occur following brain tumor surgeries.[
The incidence of cerebral vasospasm following brain tumor surgeries is not well established, but it is estimated to be exceedingly rare.[
The diagnosis of cerebral vasospasm is typically made using a combination of clinical examination, imaging studies, and blood flow measurements. Computed tomography and magnetic resonance imaging are commonly used to visualize the blood vessels and assess blood flow, while transcranial Doppler ultrasound and digital subtraction angiography can be used to measure blood flow directly.[
The treatment of cerebral vasospasm following brain tumor surgeries typically involves a combination of medical and interventional therapies. Medical management may include the use of calcium channel blockers, such as nimodipine, to relax the blood vessels, and improve blood flow. Hemodynamic support, such as maintaining adequate blood pressure and blood flow, may also be used to reduce the incidence and severity of vasospasm. In severe cases, interventional techniques, such as angioplasty, may be necessary to improve blood flow and alleviate vasospasm.[
In another study by Bejjani et al., they retrospectively studied patients with SAH who underwent angioplasty in their institution. One of their objectives was to attempt to determine if there was an association between study outcomes (clinical improvement, outcome at the time of discharge, and long-term outcome) and possible risk factors (patient age, patient sex, Hunt and Hess grade after SAH, interval, number of vessels undergoing angioplasty, day of surgery, and day of angioplasty). Using univariate analysis, they determined that the only significant association was between clinical improvement and interval (<24 h and >24 h) from deterioration to angioplasty. They found no statistically significant association between the other risk factors and any of the study outcome measures.[
To further investigate the association between clinical improvement and interval, a multivariate approach with logistic regression was used. This approach included the additional factors (patient age, patient sex, Hunt and Hess grade at admission, day of angioplasty, and number of vessels undergoing angioplasty). The results of the multivariate analysis showed that the additional factors did not add to the association between improvement and interval. The interval remained a good predictor of patient improvement. This suggests that the interval from deterioration to angioplasty is the most important factor in determining patient outcome.[
We hope that our findings will help to produce more high-quality studies that will aim to assess the best method to manage vasospasm post neurosurgery and to assess the factors that can predict the outcomes of such patients.
CONCLUSION
This systematic review provides a comprehensive overview and analysis of the management of postoperative cerebral vasospasm after brain tumor surgery. The results of this study suggest that there is no significant correlation between the outcome and the management strategy, pathology, time to develop vasospasm, age, circulation, or type of surgery. However, the data showed that the majority of cases had an incomplete recovery, with only 11 patients having a complete recovery. Cerebral vasospasm is a rare but potentially severe complication of brain tumor surgeries, and early recognition and management of this condition can improve the outcome and reverse neurological deficits. While there is no reliable way to predict the outcome of patients who develop cerebral vasospasm following brain tumor surgeries, vigilance in monitoring patients postoperatively for any signs of cerebral vasospasm is crucial. This study provides insight into the management of postoperative vasospasm after brain tumor surgery and can help guide future research and clinical practice.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Abuzayed B, Al-Abadi H, Al-Otti S, Baniyaseen K, Al-Sharki Y. Neuronavigation-guided endoscopic endonasal resection of extensive skull base mucormycosis complicated with cerebral vasospasm. J Craniofac Surg. 2014. 25: 1319-23
2. Afshari FT, Fitzgerald JJ, Higgins JN, Garnett MR, Fernandes HM, Santarius T. Diffuse cerebral vasospasm following resection of a hypoglossal schwannoma in a child. Br J Neurosurg. 2014. 28: 541-3
3. Aggarwal V, Nair P, Shivhare P, Kumar KS, Jayadevan ER, Nair S. Management of postoperative vasospasm following endoscopic endonasal surgery for craniopharyngioma: Report and review of literature. Neurol India. 2019. 67: 606-9
4. Alotaibi NM, Lanzino G. Cerebral vasospasm following tumor resection. J Neurointerv Surg. 2013. 5: 413-8
5. Amuluru K, Al-Mufti F, Singh IP, Frohman LP, Gandhi CD, Liu JK. Reversal of visual impairment associated with vasospasm after resection of sphenoclinoidocavernous meningioma with intra-arterial verapamil. World Neurosurg. 2016. 92e: 581-5
6. Aoki N, Origitano TC, Al-Mefty O. Vasospasm after resection of skull base tumors. Acta Neurochir (Wien). 1995. 132: 53-8
7. Aw D, Aldwaik MA, Taylor TR, Gaynor C. Intracranial vasospasm with delayed ischaemic deficit following epidermoid cyst resection. Br J Radiol. 2010. 83: e135-7
8. Barrow DL, Tindall GT. Loss of vision after transsphenoidal surgery. Neurosurgery. 1990. 27: 60-8
9. Bejjani GK, Bank WO, Olan WJ, Sekhar LN. The efficacy and safety of angioplasty for cerebral vasospasm after subarachnoid hemorrhage. Neurosurgery. 1998. 42: 979-86 discussion 986-77
10. Bejjani GK, Duong DH, Kalamarides M, Ziyal I, Sullivan BJ. Cerebral vasospasm after tumor resection. A case report. Neurochirurgie. 1997. 43: 164-8
11. Bierer J, Wolf A, Lee DH, Rotenberg BW, Duggal N. Bilateral caudate nucleus infarcts: A case report of a rare complication following endoscopic resection of a tuberculum sellae meningioma. Surg Neurol Int. 2017. 8: 235
12. Budnick HC, Tomlinson S, Savage J, Cohen-Gadol A. Symptomatic cerebral vasospasm after transsphenoidal tumor resection: Two case reports and systematic literature review. Cureus. 2020. 12: e8171
13. Camp PE, Paxton HD, Buchan GC, Gahbauer H. Vasospasm after trans-sphenoidal hypophysectomy. Neurosurgery. 1980. 7: 382-6
14. Cervoni L, Salvati M, Santoro A. Vasospasm following tumor removal: Report of 5 cases. Ital J Neurol Sci. 1996. 17: 291-4
15. Chang SD, Yap OW, Adler JR. Symptomatic vasospasm after resection of a suprasellar pilocytic astrocytoma: Case report and possible pathogenesis. Surg Neurol. 1999. 51: 521-6 discussion 526-7
16. Cooke-Yarborough C, Turner J, Pell M, Sheehy J. Myonecrosis of the middle cerebral artery with thrombosis and cerebral infarction following resection of meningioma. Pathology. 1993. 25: 416-9
17. de Almeida GM, Bianco E, Souza AS. Vasospasm after acoustic neuroma removal. Surg Neurol. 1985. 23: 38-40
18. Deghedy M, Pizer B, Kumar R, Mallucci C, Avula S. Basilar artery vasospasm as a cause of post-operative cerebellar mutism syndrome. Case Rep Pediatr. 2022. 2022: 9148100
19. Ecker RD, Atkinson JL, Nichols DA. Delayed ischemic deficit after resection of a large intracranial dermoid: Case report and review of the literature. Neurosurgery. 2003. 52: 706-10 discussion 709-10
20. Eseonu CI, ReFaey K, Geocadin RG, Quinones-Hinojosa A. Postoperative cerebral vasospasm following transsphenoidal pituitary adenoma surgery. World Neurosurg. 2016. 92: 7-14
21. Findlay JM, Nisar J, Darsaut T. Cerebral vasospasm: A review. Can J Neurol Sci. 2016. 43: 15-32
22. Fisher CM, Roberson GH, Ojemann RG. Cerebral vasospasm with ruptured saccular aneurysm--the clinical manifestations. Neurosurgery. 1977. 1: 245-8
23. Friedman JA, Meyer FB, Wetjen NM, Nichols DA. Balloon angioplasty to treat vasospasm after transsphenoidal surgery. Case illustration. J Neurosurg. 2001. 95: 353
24. Gocmen S, Acka G, Karaman K, Kahraman S. Cerebral vasospasm after posterior fossa tumor surgery: A case report and literature review. Pediatr Neurosurg. 2020. 55: 393-8
25. Hansen D, Hidalgo J, Cohen A, Mukherjee D, Scafidi S. Evaluation and management of symptomatic vasospasm following endoscopic endonasal resection of pediatric adamantinomatous craniopharyngioma. Case Rep Pediatr. 2020. 2020: 8822874
26. Jacob JT, Hunt CH, Wijdicks EF, Rabinstein AA, Cloft H, Link MJ. Diffuse cerebral vasospasm after resection of a posterior fossa ependymoma. Neurocrit Care. 2011. 14: 86-90
27. Karimnejad K, Sweeney JM, Antisdel JL. Cerebral vasospasm following transsphenoidal hypophysectomy in the treatment of lymphocytic hypophysitis. J Craniofac Surg. 2016. 27: 988-91
28. Kasliwal MK, Srivastava R, Sinha S, Kale SS, Sharma BS. Vasospasm after transsphenoidal pituitary surgery: A case report and review of the literature. Neurol India. 2008. 56: 81-3
29. Khan MQ, Cirjan C, Quadri N, Alexopoulos G, Coppens J. Symptomatic cerebral vasospasm in the setting of carmustine wafer placement for glioblastoma: A case presentation and review of literature. Surg Neurol Int. 2020. 11: 168
30. Kinoshita Y, Sato K, Ito T, Nakamura H. Diffuse cerebral vasospasm after removal of a posterior fossa hemangioblastoma in a 62-year-old woman. World Neurosurg. 2020. 143: 97-101
31. Krayenbuehl H. A contribution to the problem of cerebral angiospastic insult. Schweiz Med Wochenschr. 1960. 90: 961-5
32. Lee TT, Ragheb J, Bruce JC, Altman N, Morrison G. Diffuse cerebral vasospasm with ischemia after resection of a cerebellopontine angle primitive neuroectodermal tumor in a child. Pediatr Neurosurg. 1998. 29: 300-3
33. LeRoux PD, Haglund MM, Mayberg MR, Winn HR. Symptomatic cerebral vasospasm following tumor resection: Report of two cases. Surg Neurol. 1991. 36: 25-31
34. Macdonald RL, Hoffman HJ. Subarachnoid hemorrhage and vasospasm following removal of craniopharyngioma. J Clin Neurosci. 1997. 4: 348-52
35. Mawk JR, Ausman JI, Erickson DL, Maxwell RE. Vasospasm following transcranial removal of large pituitary adenomas. Report of three cases. J Neurosurg. 1979. 50: 229-32
36. Nash R, Elwell V, Brew S, Powell M, Grieve JP. Management strategy for treatment of vasospasm following transsphenoidal excision of craniopharyngioma. Acta Neurochir (Wien). 2016. 158: 2105-8
37. Page P, Kim D, Hall G, Koutourousiou M. Cerebral vasospasms following endoscopic endonasal surgery for pituitary adenoma resection in the absence of post-operative subarachnoid hemorrhage. J Neurol Stroke. 2016. 4: 00130
38. Popugaev KA, Savin IA, Lubnin AU, Goriachev AS, Kadashev BA, Kalinin PL. Unusual cause of cerebral vasospasm after pituitary surgery. Neurol Sci. 2011. 32: 673-80
39. Prontera A, Puzzolante A, Carpeggiani P, Pavesi G. Symptomatic anterior cerebral artery vasospasm after brainstem hemangioblastoma resection. A case report. Neuroradiol J. 2014. 27: 186-90
40. Puri AS, Zada G, Zarzour H, Laws E, Frerichs K. Cerebral vasospasm after transsphenoidal resection of pituitary macroadenomas: Report of 3 cases and review of the literature. Neurosurgery. 2012. 71: 173-80 discussion 180-71
41. Qi J, Zhang L, Jia W, Zhang J, Wu Z. Diffuse cerebral vasospasm after resection of schwannoma: A case report. Neuropsychiatr Dis Treat. 2015. 11: 317-20
42. Rao VK, Haridas A, Nguyen TT, Lulla R, Wainwright MS, Goldstein JL. Symptomatic cerebral vasospasm following resection of a medulloblastoma in a child. Neurocrit Care. 2013. 18: 84-8
43. Ricarte IF, Funchal BF, Alves MA, Gomes DL, Valiente RA, Carvalho FA. Symptomatic cerebral vasospasm and delayed cerebral ischemia following transsphenoidal resection of a craniopharyngioma. J Stroke Cerebrovasc Dis. 2015. 24: e271-3
44. Salunke P, Sodhi HB, Aggarwal A, Ahuja CK. Delayed cerebral vasospasm following surgery for craniopharyngioma. J Neurosci Rural Pract. 2013. 4: 107-9
45. Santarius T, Jian BJ, Englot D, McDermott MW. Delayed neurological deficit following resection of tuberculum sellae meningioma: Report of two cases, one with permanent and one with reversible visual impairment. Acta Neurochir (Wien). 2014. 156: 1099-102
46. Satpathy DK, Mitra C, Samal P, Pattnaik SB, Mohanty BB, Acharya SD. Delayed cerebral infarction following transsylvian surgery for craniopharyngioma presenting as status epilepticus. Neurol India. 2020. 68: 1428-30
47. Suero Molina E, Di Somma A, Stummer W, Briganti F, Cavallo LM. Clinical vasospasm after an extended endoscopic endonasal approach for recurrent pituitary adenoma: Illustrative case and systematic review of the literature. World Neurosurg. 2019. 128: 29-36
48. Webb AJ, Gillies MJ, Cadoux-Hudson TA. Acute vasospasm following transcallosal resection of a xanthogranulomatous colloid cyst of the 3rd ventricle. Clin Neurol Neurosurg. 2010. 112: 512-5