- Department of Neurosurgery, Universitas Sumatera Utara, Medan, North Sumatera, Indonesia
- School of Medicine, Universitas Sumatera Utara, Medan, North Sumatera, Indonesia.
Correspondence Address:
Andre Marolop Pangihutan Siahaan, Department of Neurosurgery, Universitas Sumatera Utara, Medan, Indonesia.
DOI:10.25259/SNI_715_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Andre Marolop Pangihutan Siahaan1, Bahagia Willibrordus Maria Nainggolan2, Martin Susanto2, Rr Suzy Indharty1, Steven Tandean1. Managing the “big black brain” in low resource setting: A case report of early outcome after hinge craniotomy. 15-Dec-2023;14:427
How to cite this URL: Andre Marolop Pangihutan Siahaan1, Bahagia Willibrordus Maria Nainggolan2, Martin Susanto2, Rr Suzy Indharty1, Steven Tandean1. Managing the “big black brain” in low resource setting: A case report of early outcome after hinge craniotomy. 15-Dec-2023;14:427. Available from: https://surgicalneurologyint.com/surgicalint-articles/12673/
Abstract
Background: The big black brain (BBB) phenomenon is described as an infant’s response to an acute subdural hematoma (SDH). It is characterized by hypodensity and swelling of the supratentorial compartment as a whole. Numerous factors may contribute to the formation of the BBB. Due to its high morbidity and mortality, the management of BBB is still debatable. In this report, we describe a 2-month-old boy who had bilateral hemispheric hypodensity and underwent hinge craniotomy.
Case Description: The patient was referred to our hospital with decreased consciousness. The patient had a history of seizures and cardiopulmonary arrest. There is no history of trauma. The computed tomography revealed a subacute SDH on the left parietal and occipital lobe along with hypodensity in both hemispheres with preservation of posterior fossa, consistent with hemispheric hypodensity. We performed a hinge craniotomy for the emergency procedure and evacuated only the hemisphere with the bleeding side. The patient cried spontaneously 24 hours after the procedure and was discharged six days later.
Conclusion: Early outcomes of hinge craniotomy as an alternative procedure for treating the BBB were positive. However, long-term outcomes, particularly the infant’s development, should be monitored.
Keywords: Big black brain, Hinge craniotomy, Infants, Outcome
INTRODUCTION
The big black brain (BBB) phenomenon is described as a response to acute subdural hematoma (SDH) in infants.[
Hinge craniotomy is a technique that permits a degree of decompression while retaining the bone flap in situ, in a “floating” or “hinged” fashion, and maintains cerebral protection while reducing postoperative complications and eliminating the need for subsequent cranioplasty procedures.[
CASE DESCRIPTION
A 2-month-old boy was referred to our hospital with decreasing consciousness. His mother had noticed that the patient had “ordinary cold symptoms” 5 days prior that were escalating gradually. The child had a mild fever and was not doing well at first, but her mother noted that the patient had become paler than before and had a seizure on the 3rd day. The seizure lasted around 2 min on the left side of the body and ended without any intervention. The patient was subsequently taken to a primary healthcare service nearby. Anemia (hemoglobin) 6.1 g/dL, hematocrit 15.8%, leukocyte 14.000/L, and thrombocyte 450.000/L were discovered during the initial laboratory evaluation. There was no history of trauma, and according to the mother, the child was in a healthy condition before. The patient was referred to our hospital after receiving a loading dose of phenytoin.
The paramedic reported that the patient had stopped breathing en route to our hospital’s emergency department. The patient was unconscious and had apnea, with a heart rate of 80 beats/min, according to the initial assessment. Intubation and stabilization were started as an emergency treatment. Following intubation, the vital signs were as follows: blood pressure 124/72 mmHg, heart rate 78 beats/ min, and oxygen saturation on a ventilator of 97%. There was no sign of trauma to the head or any other area of the body. The main fontanelle, on the other hand, was bulging and tight, with a sluggish-symmetric pupil (2 mm). Following painful stimuli, the child was decorticated with minimum flexion. A non-contrast head CT scan revealed a subacute SDH on the left parietal and occipital lobe along with hypodensity in both hemispheres with preservation of posterior fossa, consistent with hemispheric hypodensity (the “big black brain”) [
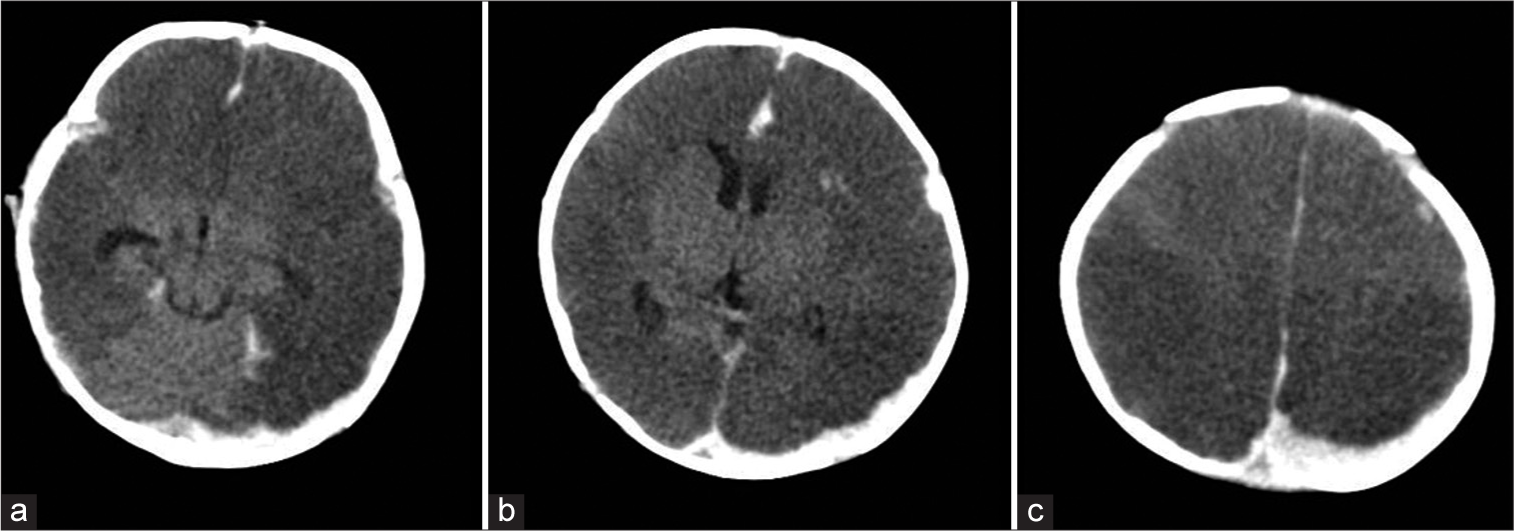
Figure 1:
Non-contrast computed tomography scan. (a) Bilateral hemispheric hypodensity with preservation of posterior fossa was seen, consistent with a “big black brain” (b) Preservation of central nuclei was also seen, along with a left hemispheric crescentic lesion, suggestive of a subdural hematoma. (c) The hypodense lesion was also seen up to the vertex region.
To treat the anemia and prolonged hemorrhagic screening test, packed red cells and fresh frozen plasma transfusions were started within the first 48 h. We performed a hinge craniotomy and subdural blood clot removal after stabilization [
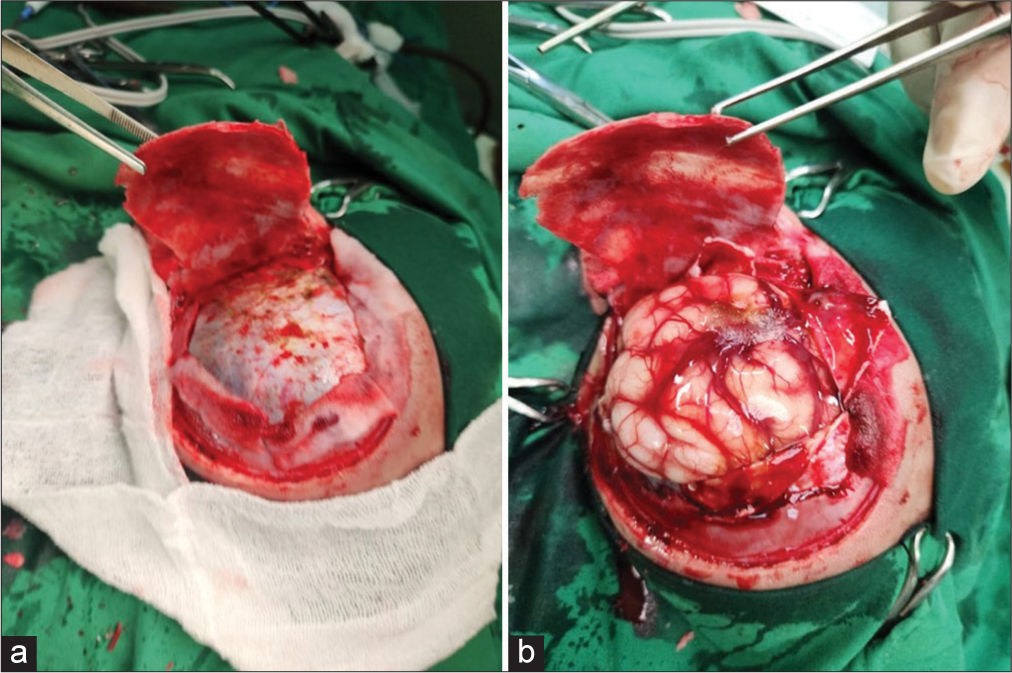
Figure 2:
Hinge craniotomy. (a) After a “three-sided craniotomy,” periosteal on the temporal base was preserved. A craniectomy on the temporal base was performed to improve the decompression size. A blue dura mater was seen. (b) After subdural blood clot removal, the brain was bulging with engorgement of the cortical vein. The bone was then left at the initial site without any fixation to maintain a “hinged” position.
Following the operation, the patient’s condition significantly improved. The patient had spontaneous breathing for the first 24 hours after the operation and was then extubated. The patient cried spontaneously the next day, and the nasogastric tube was withdrawn on the 5th postoperative day. The patient was released from the hospital on the 6th day. The patient was active two months after discharge and had no seizures. However, two months following the operation, a control CT scan indicated substantial brain atrophy in both hemispheres [
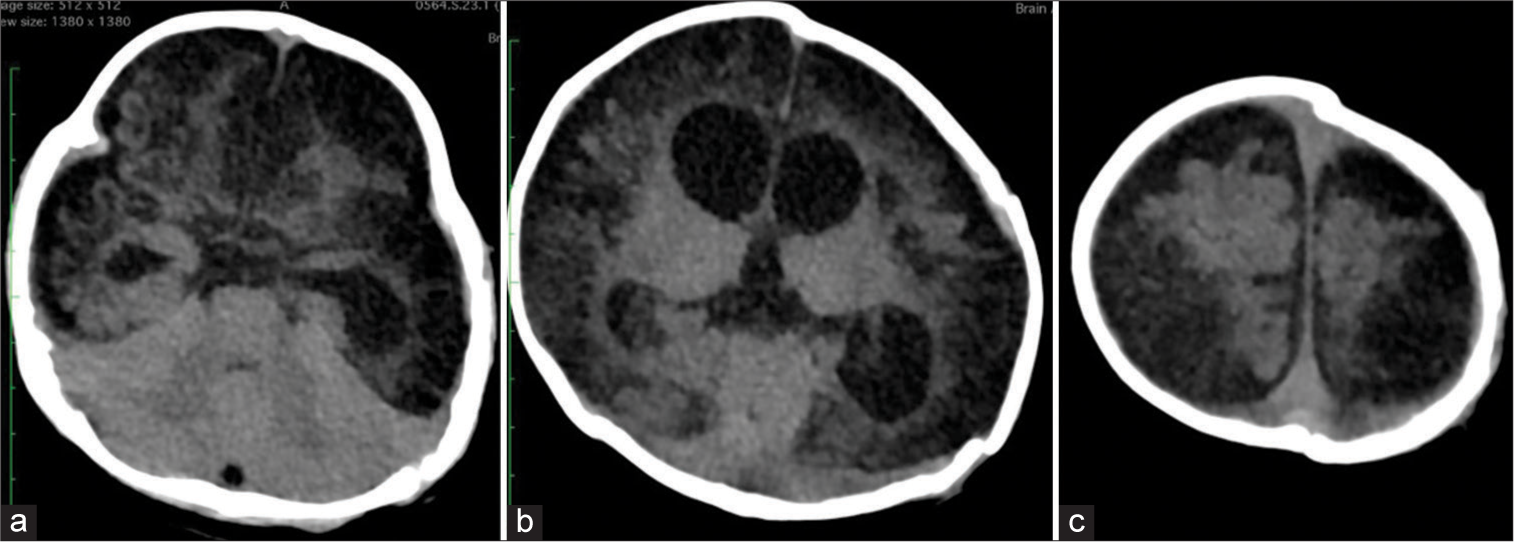
Figure 3:
Post-operative non-contrast head computed tomography scan two months following surgery. (a) There was an extensive hypodense lesion on the cortical region, as well as dilatation of the temporal horn (b) The extensive hypodense lesion on the cortical was also seen along with dilatation of the lateral ventricle. “Hinged” bone could be found on the left side. (c) The cortical hypodense lesion was also identified up to the vertex region, but relatively less than the preoperative finding.
DISCUSSION
BBB was first described by Duhaime et al. as a hemispheric hypodense associated with subdural hemorrhage.[
Our patient presented with a history of cardiopulmonary arrest (CPA) and seizures. A study outlined four pathological conditions in which CPA arrest can occur and be manifested in infants.[
Foster et al. reported intracranial hypertension and ICP monitoring intensity.[
A retrospective study found that the hinge technique effectively prevents procedure-related morbidity as well as the need for a second incision to explant the bone flap and surgery to restore cranial contour.[
CONCLUSION
The management of the big black brain is still debatable, especially in infants. Early outcomes of hinge craniotomy were reported to be favorable in this case report; however, long-term outcomes, particularly the development of the infant, should be monitored.
Ethical approval
Not applicable
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Beriwal N, Misko AL, Duhaime AC. Big black brain phenomenon: Understanding clinicoradiological dissociation in non-accidental traumatic brain injury in children. Cureus. 2020. 12: e8011
2. Costine-Bartell BA, McGuone D, Price G, Crawford E, Keeley KL, Munoz-Pareja J. Development of a model of hemispheric hypodensity (“Big Black Brain”). J Neurotrauma. 2019. 36: 815-33
3. Cox LA. The shaken baby syndrome: Diagnosis using CT and MRI. Radiol Technol. 1996. 67: 513-20
4. Dewan MC, Rattani A, Fieggen G, Arraez MA, Servadei F, Boop FA. Global neurosurgery: The current capacity and deficit in the provision of essential neurosurgical care. Executive Summary of the Global Neurosurgery Initiative at the Program in Global Surgery and Social Change. J Neurosurg. 2018. 130: 1055-64
5. Duhaime AC, Bilaniuk L, Zimmerman R The. “big black brain”: Radiographic changes after severe inflicted head injury in infancy. J Neurotrauma. 1993. 10: S59
6. Duhaime AC, Durham S. Traumatic brain injury in infants: The phenomenon of subdural hemorrhage with hemispheric hypodensity (“Big Black Brain”). Prog Brain Res. 2007. 161: 293-302
7. Foster KA, Recker MJ, Lee PS, Bell MJ, Tyler-Kabara EC. Factors associated with hemispheric hypodensity after subdural hematoma following abusive head trauma in children. J Neurotrauma. 2014. 31: 1625-31
8. Kenning TJ, Gandhi RH, German JW. A comparison of hinge craniotomy and decompressive craniectomy for the treatment of malignant intracranial hypertension: Early clinical and radiographic analysis. Neurosurg Focus. 2009. 26: E6
9. Kenning TJ, Gooch MR, Gandhi RH, Shaikh MP, Boulos AS, German JW. Cranial decompression for the treatment of malignant intracranial hypertension after ischemic cerebral infarction: Decompressive craniectomy and hinge craniotomy. J Neurosurg. 2012. 116: 1289-98
10. Ko K, Segan S. In situ hinge craniectomy. Neurosurgery. 2007. 60: 255-8
11. Lassen B, Helseth E, Egge A, Due-Tønnessen BJ, Rønning P, Meling TR. Surgical mortality and selected complications in 273 consecutive craniotomies for intracranial tumors in pediatric patients. Neurosurgery. 2012. 70: 936-43
12. Layard Horsfall H, Mohan M, Devi BI, Adeleye AO, Shukla DP, Bhat D. Hinge/floating craniotomy as an alternative technique for cerebral decompression: A scoping review. Neurosurg Rev. 2020. 43: 1493-507
13. Le TH, Gean AD. Neuroimaging of traumatic brain injury. Mt Sinai J Med. 2009. 76: 145-62
14. Lee Y, Lee KS, Hwang DH, Lee IJ, Kim HB, Lee JY. MR imaging of shaken baby syndrome manifested as chronic subdural hematoma. Korean J Radiol. 2001. 2: 171-4
15. Luyet FM, Feldman KW, Knox BL. The big black brain: Subdural hemorrhage with hemispheric swelling and low attenuation. J Child Adolesc Trauma. 2018. 11: 241-7
16. Momose H, Sorimachi T, Aoki R, Atsumi H, Matsumae M. Cerebral infarction following acute subdural hematoma in infants and young children: Predictors and significance of FLAIR vessel hyperintensity. Neurol Med Chir (Tokyo). 2015. 55: 510-8
17. Okuma Y, Yasuhara T, Kin I, Daido S, Date I. Inverted gull-wing hinge decompressive craniotomy for infantile acute subdural hematoma: A case report. Brain Circ. 2023. 9: 35-8
18. Paek D, Kwon DI. A review on four different paths to respiratory arrest from brain injury in children; implications for child abuse. J Forensic Leg Med. 2020. 71: 101938
19. Park YS. Complex pathophysiology of abusive head trauma with poor neurological outcome in infants. J Korean Neurosurg Soc. 2022. 65: 385-96
20. Rocque BG, Agee BS, Thompson EM, Piedra M, Baird LC, Selden NR. Complications following pediatric cranioplasty after decompressive craniectomy: A multicenter retrospective study. J Neurosurg Pediatr. 2018. 22: 225-32
21. Schmidt JH, Reyes BJ, Fischer R, Flaherty SK. Use of hinge craniotomy for cerebral decompression. Technical note. J Neurosurg. 2007. 107: 678-82
22. Wicaksono AS, Tamba DA, Sudiharto P, Basuki E, Pramusinto H, Hartanto RA. Neurosurgery residency program in Yogyakarta, Indonesia: Improving neurosurgical care distribution to reduce inequality. Neurosurg Focus. 2020. 48: E5
23. Yokota H, Sugimoto T, Nishiguchi M, Hashimoto H. Greenstick fracture-hinge decompressive craniotomy in infants: Illustrative case and literature review of techniques for decompressive craniotomy without bone removal. Childs Nerv Syst. 2019. 35: 1491-7
24. Yoshimura J, Morikawa M, Kumagai S, Saito T, Takahashi H, Fujiwara H. Surgical case of accidental infantile acute subdural hematoma caused by household minor head trauma: Hyperperfusion during postoperative hemispheric hypodensity, namely “big black brain”. No Shinkei Geka. 2020. 48: 841-7
25. Zhao J, Hylin MJ, Kobori N, Hood KN, Moore AN, Dash PK. Post-injury administration of galantamine reduces traumatic brain injury pathology and improves outcome. J Neurotrauma. 2018. 35: 362-74