- Department of Neurosurgery, Cedars-Sinai Medical Center, Los Angeles, United States.
- Department of Neurosurgery, University of Florida College of Medicine, Gainesville, United States.
- Department of Pathology and Laboratory Medicine, Cedars-Sinai Medical Center, Los Angeles, United States.
Correspondence Address:
Michelot Michel, Department of Neurosurgery, Cedars-Sinai Medical Center, Los Angeles, United States.
DOI:10.25259/SNI_599_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Andre Everett Boyke1, Michelot Michel1,2, Catherine Michelle Garcia1, Serguei I. Bannykh3, Julie Lynn Chan1, Keith L. Black1. Meningioma transformation to glioblastoma following stereotactic radiosurgery: A case report and review of the literature. 13-Oct-2023;14:364
How to cite this URL: Andre Everett Boyke1, Michelot Michel1,2, Catherine Michelle Garcia1, Serguei I. Bannykh3, Julie Lynn Chan1, Keith L. Black1. Meningioma transformation to glioblastoma following stereotactic radiosurgery: A case report and review of the literature. 13-Oct-2023;14:364. Available from: https://surgicalneurologyint.com/surgicalint-articles/12593/
Abstract
Background: Meningiomas are the most common primary intracranial tumor with increasing incidence. Stereotactic Radiosurgery Gamma Knife (SRS-GK) is a commonly used modality for neoadjuvant and adjuvant treatment of these tumors and is often necessary for long-term disease control, particularly for the World Health Organization grade II/III meningiomas. While there is strong evidence to support the use of SRS-GK for meningioma, there exists a risk of secondary malignancy that is not well understood. We report a case of glioblastoma (GBM) that arose near the bed of a meningioma previously treated with SRS-GK and discuss other cases of GBM that emerged at a site of meningioma reported in the literature.
Case Description: A 79-year-old female with a history of a blood-clotting disorder presented to the hospital with sudden facial sensory disturbances. On magnetic resonance imaging (MRI), a homogeneously enhancing lesion was observed in the right temporal lobe, consistent with a meningioma. Following 2 years of surveillance, the patient underwent SRS-GK for enlargement of the lesion. The patient later presented with headache and gait instability 12 years following SRS-GK. MRI revealed a large ring-enhancing lesion with surrounding edema histologically confirmed to be a GBM. At 9 months following initial tumor resection and a combination of radiotherapy and temozolomide, the patient was neurologically intact.
Conclusion: There is a very small risk of meningioma to GBM conversion following SRS. Although SRS-GK poses a risk of secondary malignancy, there are some reported cases that underwent malignant transformation without SRS-GK. This suggests that SRS-GK is not the only factor in transformation and is a reasonable therapeutic modality to consider utilizing. Patients and their families should be appropriately counseled on the potential risks of radiation therapy, even for benign lesions like a meningioma.
Keywords: Gamma knife, Glioblastoma multiforme, Malignant complication, Meningioma, Stereotactic radiosurgery
INTRODUCTION
Meningiomas and glioblastoma (GBM) are among the most common primary benign and malignant brain tumors, respectively.[
CASE PRESENTATION
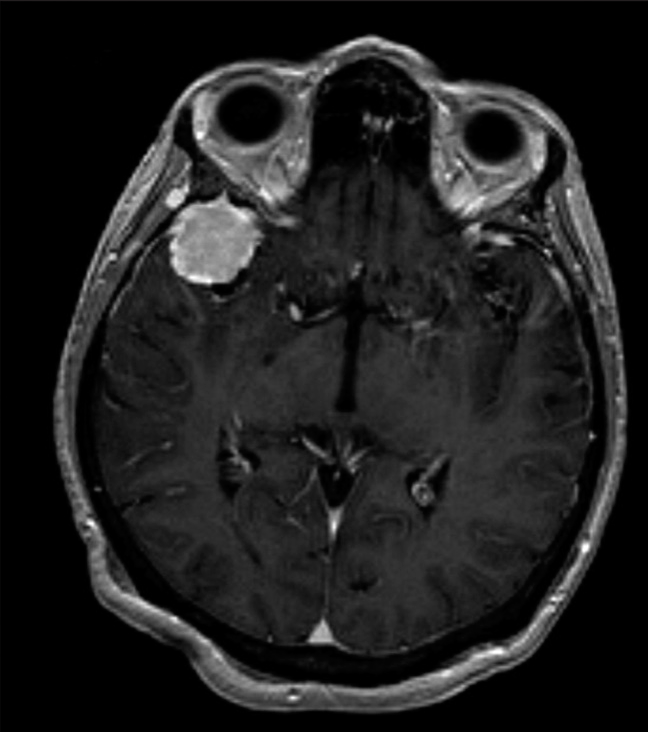
A 79-year-old female with a medical history of Factor V Leiden (FVL) and venous thromboembolism (VTE) presented with a sudden bursting sensation above the right eye. Magnetic resonance imaging (MRI) of the brain revealed a homogeneously enhancing 2.0 × 2.1 × 1.9 cm lesion in the right temporal lobe and right sphenoid wing, consistent with a meningioma [
Figure 2:
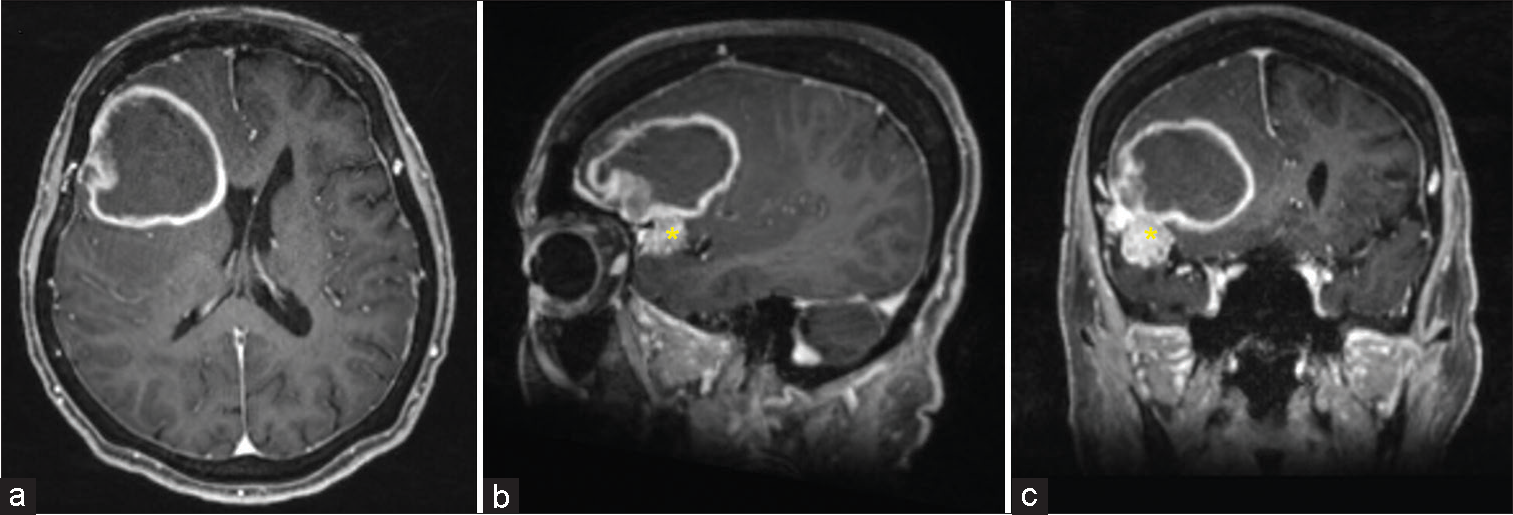
(a) Axial, (b) sagittal, (c) coronal views of brain magnetic resonance imaging with gadolinium 12 years post gamma knife radiosurgery depicting an emergence of a ring-enhancing lesion adjacent to unevenly enhancing meningioma of the Sylvian fissure (asterisks of b and c). The meningioma remained stable or slightly decreased in size.
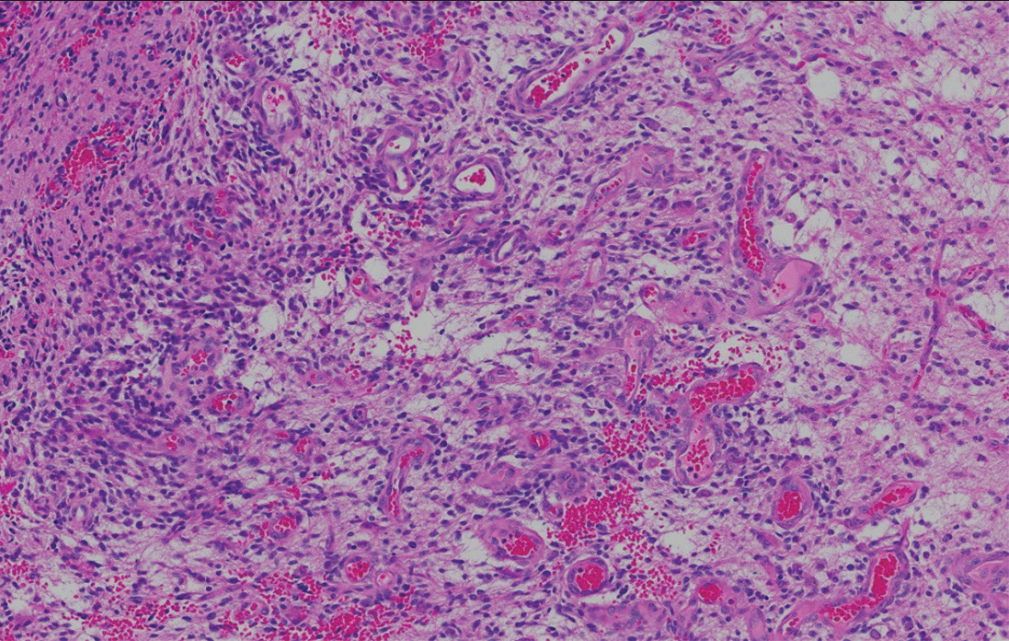
Before elective scheduled surgery, she was placed on a heparin drip given her history of FVL and VTE. Two days following the heparin drip initiation, the patient developed a headache with nausea and emesis. A non-contrast head computed tomography was obtained and showed significant right frontal hemorrhage, increased edema, and worsened midline shift up to 16 mm. She was taken emergently to the operating room for hematoma evacuation. Following careful removal of the hematoma, areas of the lesion were biopsied. Pathologic analysis revealed isocitrate dehydrogenase 1 and 2 wild-type the World Health Organization (WHO) grade IV GBM multiforme with intact retained α thalassemia/mental retardation syndrome X-linked (ATRX), loss of NF1 gene, and combined losses of cyclin-dependent kinase inhibitor A and B. Deoxyribonucleic acid repair gene methylguanine methyltransferase showed promoter methylation [
She underwent adjuvant fractionated radiotherapy with a dose of 34 Gy with concurrent chemotherapy with temozolomide. At the 6-month follow-up, tumor progression was noted, and the patient underwent a repeat right frontal craniotomy for debulking of tumor. At the latest documented follow-up (9 months following initial craniotomy for hemorrhagic GBM; and 3 months following repeat craniotomy), the patient was neurologically intact with a Karnofsky performance score of 100.
Methods
We conducted a comprehensive literature search of electronic online databases (PubMed/MEDLINE) to identify relevant case reports or studies published up to March 2023. A combination of keywords and MeSH terms related to the transformation of meningioma and radiation therapy was used to identify relevant articles. All articles published in the English language were included in the study. Inclusion criteria required the initial lesion to be a meningioma and the secondary lesion to be a GBM originating from a similar or adjacent anatomical location as the primary meningioma. Exclusion criteria included the co-existence of both distinct lesions at the time of initial presentation.
RESULTS
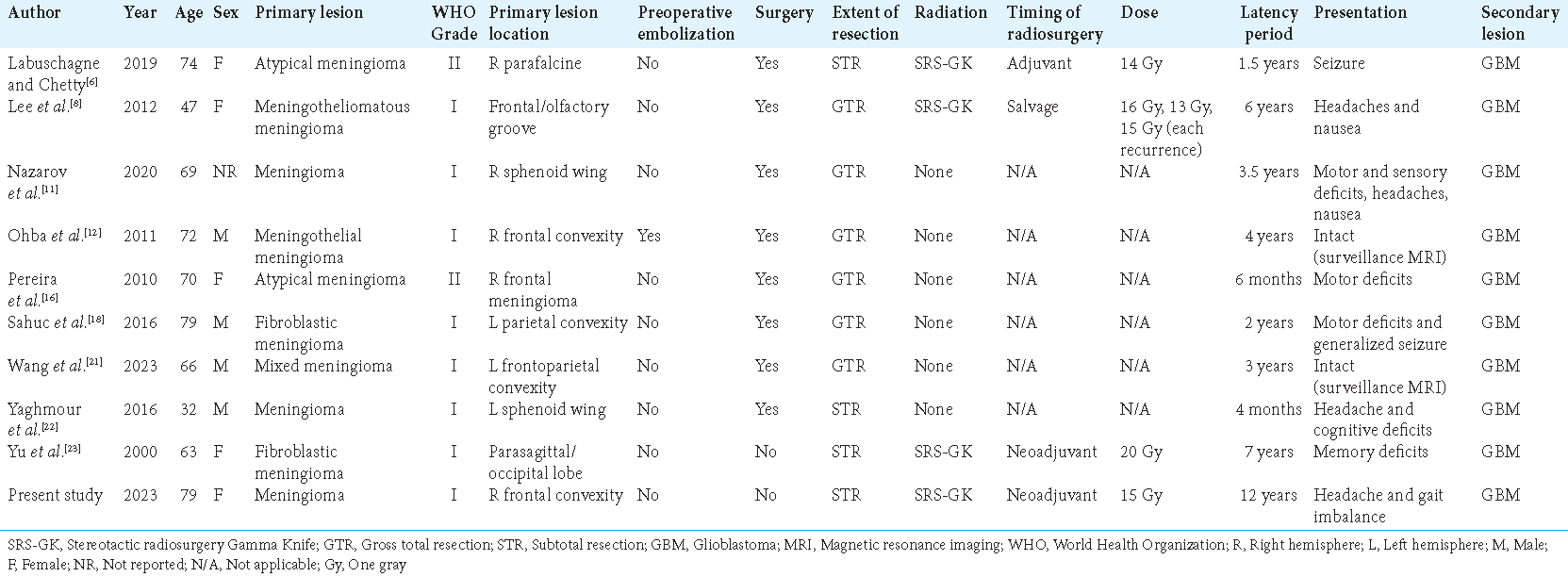
Nine case reports describing the transformation of meningioma to malignant high-grade glioma GBM were identified [
DISCUSSION
SRS is a vital and commonly used tool in the management of both benign and malignant intracranial tumors. Patients with clinical profiles well suited for SRS often stand to benefit from these procedures either as an alternative to more invasive surgery or as a means of continued disease control following surgical resection. Our current patient was initially managed with SRS-GK with a dose of 15 Gy instead of craniotomy for probable meningioma based on MR imaging. The presence of a WHO Grade IV GBM extending from the site of the originally radiated lesion after a 12-year latency period makes this likely an SRS-induced GBM, successfully satisfying the first two criteria employed by Cahan et al. to define a radiation-induced secondary malignancy.[
Radiation-induced glioma is rare and has an incidence rate of ~3% within 15 years of exposure.[
Although the co-occurrence of meningioma and GBM is exceedingly rare, the incidence of both tumors is increasing.[
Moreover, the previous reports have established the ability of tumors to dedifferentiate into a less differentiated state.[
Overall, our findings suggest that there is a very small potential for meningioma transformation to a GBM following initial treatment. Although SRS-GK poses a risk of secondary malignancy, we report several cases of initial lesions that underwent malignant transformation without SRS-GK and as such should not be a significant reason not to utilize this therapeutic modality.
CONCLUSION
The increased use of SRS-GK in the management of benign tumors may mean that neurosurgeons will encounter more radiation-induced secondary lesions requiring further surgical and medical intervention. In clinical scenarios where SRS-GK is employed as an alternative to surgical resection, longer-term surveillance beyond a 5-year mark could be considered. This becomes particularly important in the absence of a tissue-based diagnosis following resection that can rule out the coexistence of more aggressive or cancerous cells. Long-term follow-up and appropriate patient counseling are imperative following initial tumor resection and/or radiation therapy, even in initially benign lesions such as meningioma.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
This work was supported by the Ray Charles Foundation Scholarship in Neurosurgery Fund.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The author(s) confirms that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Cahan WG, Woodard HQ, Higinbotham NL, Stewart FW, Coley BL. Sarcoma arising in irradiated bone: Report of eleven cases 1948. Cancer. 1998. 82: 8-34
2. Chen B, Chen C, Zhang Y, Xu J. Recent incidence trend of elderly patients with glioblastoma in the United States, 2000–2017. BMC Cancer. 2021. 21: 54
3. Coskun S, Coskun A, Gursan N, Aydin MD. Post-traumatic glioblastoma multiforme: A case report. Eurasian J Med. 2011. 43: 50-3
4. Friedmann-Morvinski D, Verma IM. Dedifferentiation and reprogramming: Origins of cancer stem cells. EMBO Rep. 2014. 15: 244-53
5. Garcia-Segura ME, Erickson AW, Jairath R, Munoz DG, Das S. Necrosis and brain invasion predict radio-resistance and tumor recurrence in atypical meningioma: A retrospective cohort study. Neurosurgery. 2020. 88: E42-8
6. Labuschagne JJ, Chetty D. Glioblastoma multiforme as a secondary malignancy following stereotactic radiosurgery of a meningioma: Case report. Neurosurg Focus. 2019. 46: E11
7. Lapointe S, Perry A, Butowski NA. Primary brain tumours in adults. Lancet. 2018. 392: 432-46
8. Lee HS, Kim JH, Lee JI. Glioblastoma following radiosurgery for meningioma. J Korean Neurosurg Soc. 2012. 51: 98-101
9. Momin AA, Shao J, Soni P, Almeida JP, Suh JH, Murphy ES. Outcomes of salvage radiation for recurrent world health organization grade II meningiomas: A retrospective cohort study. J Neurooncol. 2021. 152: 373-82
10. Munjal S, Kumar J, Jain S, Mehta VS. Glioma simultaneously present with adjacent meningioma: Case report and literature review. Asian J Neurosurg. 2019. 14: 272-4
11. Nazarov VV, Linde NN, Kim DS, Danilov GV, Cherekaev VA, Kozlov AV. Glioblastoma in the region of previously resected meningioma. Case report and literature review. Zh Vopr Neirokhir Im N N Burdenko. 2020. 84: 61-8
12. Ohba S, Shimizu K, Shibao S, Miwa T, Nakagawa T, Sasaki H. A glioblastoma arising from the attached region where a meningioma had been totally removed. Neuropathology. 2011. 31: 606-11
13. Osipov V, Ho KC, Krouwer HG, Meyer G, Shidham VB. Post-radiation dedifferentiation of meningioma into osteosarcoma. BMC Cancer. 2002. 2: 34
14. Ostrom QT, Cioffi G, Gittleman H, Patil N, Waite K, Kruchko C. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2012–2016. Neuro Oncol. 2019. 21: v1-100
15. Pallud J, Capelle L, Taillandier L, Badoual M, Duffau H, Mandonnet E. The silent phase of diffuse low-grade gliomas. Is it when we missed the action? Acta Neurochir (Wien) 2013. ;. 155: 2237-42
16. Pereira EA, Dabbous B, Qureshi HU, Ansorge O, Bojanic S. Rapid development of glioblastoma at the site of atypical meningioma resection. Br J Neurosurg. 2010. 24: 471-3
17. Prasad G, Haas-Kogan DA. Radiation-induced gliomas. Expert Rev Neurother. 2009. 9: 1511-7
18. Sahuc P, Joubert C, Nguyen AT, Fouet B, Wybrecht D, Faivre A. Glioblastoma secondary to meningioma: A case report and literature review. World Neurosurg. 2017. 98: 881.e9-13
19. Shintaku M, Adachi Y, Arai A, Koyama J. Anaplastic and meningothelial meningiomas in a single tumor: A “dedifferentiated meningioma”?. Neuropathology. 2016. 36: 584-90
20. Singh GK, Yadav V, Singh P, Bhowmik KT. Radiation-induced malignancies making radiotherapy a “two-edged sword”: A review of literature. World J Oncol. 2017. 8: 1-6
21. Wang Z, Sun S, Xie K, Miao J. Glioblastoma in the contralateral cerebral hemisphere with previous surgery for meningioma: A case report. Medicine (Baltimore). 2023. 102: e32616
22. Yaghmour W, Kurdi ME, Baeesa SS. De novo glioblastoma in the territory of a recent middle cerebral artery infarction and a residual meningioma: Pathogenesis revisited. World J Surg Oncol. 2016. 14: 112
23. Yu JS, Yong WH, Wilson D, Black KL. Glioblastoma induction after radiosurgery for meningioma. Lancet. 2000. 356: 1576-7
24. Zhang Z, Yang Y, Zhang K, Zhuang J, Shao F, Liu H. Collision tumor of glioblastoma and meningioma: Case report and literature review. World Neurosurg. 2018. 117: 137-41