- College of Medicine, Qassim University, Qassim,
- College of Medicine, King Saud bin Abdulaziz University for Health Sciences,
- King Abdullah International Medical Research Center,
- Division of Neurosurgery, Department of Surgery, King Abdulaziz Medical City, Ministry of National Guard-Health Affairs,
- Department of Pathology and Laboratory Medicine, King Abdulaziz Medical City, Ministry of National Guard-Health Affairs, Riyadh, Saudi Arabia.
Correspondence Address:
Ali Alkhaibary, College of Medicine, King Saud bin Abdulaziz University for Health Sciences, Riyadh, Saudi Arabia.
DOI:10.25259/SNI_122_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Abdulaziz Alanazi1, Ali Alkhaibary2,3,4, Sami Khairy2,3,4, Fahd Al Sufiani3,5, Ali H. Alassiri2,3,5, Ahmed Aloraidi2,3,4, Ahmed Alkhani3,4. Metastatic brain lesion as the initial presentation of follicular thyroid carcinoma. 25-Mar-2022;13:109
How to cite this URL: Abdulaziz Alanazi1, Ali Alkhaibary2,3,4, Sami Khairy2,3,4, Fahd Al Sufiani3,5, Ali H. Alassiri2,3,5, Ahmed Aloraidi2,3,4, Ahmed Alkhani3,4. Metastatic brain lesion as the initial presentation of follicular thyroid carcinoma. 25-Mar-2022;13:109. Available from: https://surgicalneurologyint.com/surgicalint-articles/11479/
Abstract
Background: Metastatic brain lesions, of thyroid origin, are rare manifestations of differentiated thyroid cancer, with papillary thyroid carcinoma being the most common subtype. Considering the rarity of metastatic follicular thyroid carcinoma to the brain, the present article outlines its clinical presentation, neuroradiological findings, pathological features, and outcome.
Case Description: A 52-year-old female presented with a 6-month history of progressive and holocephalic headache. Examination revealed a tracheal deviation to the left side due to an enlarged goiter. Brain CT scan showed a right occipital, slightly hyperdense lesion associated with a 0.4 cm midline shift to the left side. Brain MRI demonstrated a right occipital, avidly-enhancing, extra-axial lesion with disproportionate and extensive vasogenic edema. As the lesion was solitary, the patient underwent craniotomy and tumor resection. Histopathological examination revealed a tumor consistent of small follicles, composed of uniform round nuclei without papillary thyroid carcinoma nuclear features, suggestive of metastatic follicular thyroid carcinoma to the brain. Postoperatively, the patient was neurologically intact. She was discharged in a stable condition with laboratory/ radiological investigations and follow-up at neurosurgery, endocrine, radiotherapy, and thyroid surgery clinics.
Conclusion: Follicular thyroid carcinoma may rarely metastasize to the central nervous system. A high index of suspicion is required to identify patients with thyroid cancer who initially present with neurological manifestations. Complete surgical resection of the metastatic brain lesion is safe, feasible and is associated with a prolonged overall survival.
Keywords: Central nervous system, Metastasis, Thyroid cancer
INTRODUCTION
The incidence of thyroid cancer has increased significantly in the past few decades.[
Metastatic brain lesions, of thyroid origin, are rare manifestations of differentiated thyroid cancer, with papillary thyroid cancer being the most common subtype.[
Considering the rarity of metastatic follicular thyroid carcinoma to the brain, the present article outlines its clinical presentation, neuroradiological findings, pathological features, and outcome. A review of the pertinent literature is additionally discussed.
CASE DESCRIPTION
Clinical presentation
A 52-year-old female, known to have hypertension, diabetes mellitus, and dyslipidemia, presented to the emergency department complaining of a 6-month history of progressive headache. The headache was continuous, band-like, holocephalic, and more intense in the morning. It was associated with dizziness and vomiting for 3 days before presentation. The patient reported no history of heat/cold intolerance, palpitations, dysphagia, dysphonia, or symptoms suggestive of hyper/hypometabolism.
Physical examination
On initial assessment, the patient was hypertensive, alert, and oriented to person, place, and time with a Glasgow Coma Scale of 15/15. The muscle power and sensation were intact. The cranial nerves and cerebellar examination were unremarkable. The visual fields were intact to confrontation with full extraocular muscles movement. The patient had a left-sided tracheal deviation due to an enlarged goiter.
Radiological imaging
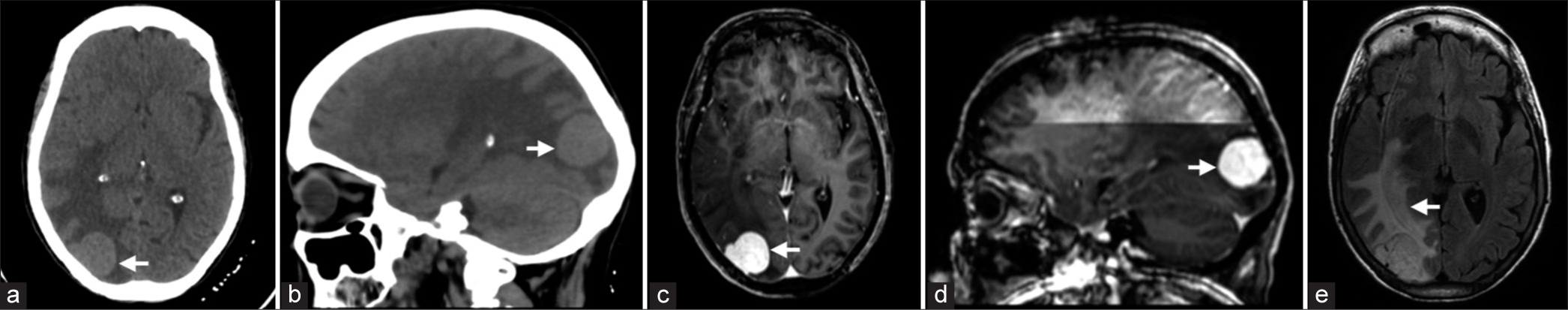
Brain computed tomography (CT) scan showed a right occipital homogenously hyperdense lesion causing 0.4 cm midline shift to the left side [
Figure 1:
(a and b) Axial and sagittal brain CT without contrast. (c and d) Axial and sagittal T1-weighted brain MRI post gadolinium administration. (e) T2/Fluid-attenuated inversion recovery brain MRI. (a and b) The images demonstrate a right occipital, extra-axial, well-defined, round hyperdense lesion, measuring 3 × 2.4 × 2.6 cm in transverse, anteroposterior, and craniocaudal dimensions (Arrow). (c and d) The lesion is dural-based and demonstrates homogeneous enhancement (Arrow) post gadolinium administration. There is no intratumoral hemorrhage. (e) There is extensive and disproportionate vasogenic edema (Arrow) involving the right occipital, temporal, and parietal lobes, causing a midline shift of 4 mm.
Surgical intervention
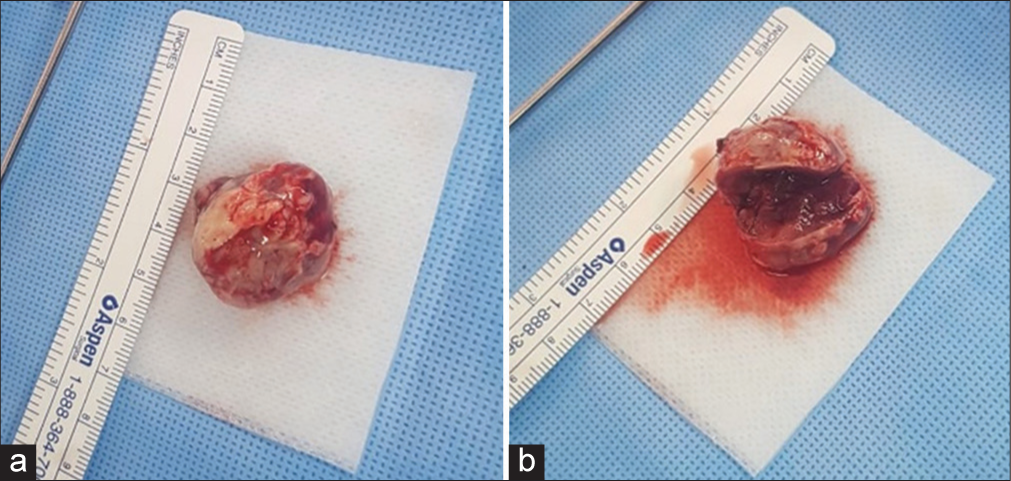
As the lesion was solitary and exerting midline shift, the patient underwent craniotomy and tumor resection. Intraoperatively, the incision was made in the parieto-occipital area. The boarders of the tumor were precisely localized using the neuronavigation system. The tumor was dural-based. Considering the well-encapsulated nature of the tumor, it was excised in one piece [
Histopathological features
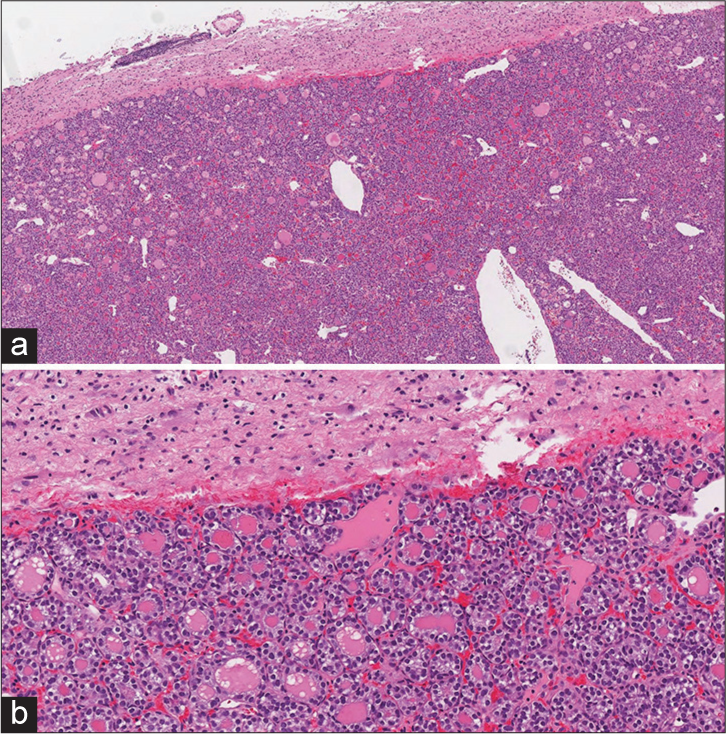
The histopathological sections of the lesion were composed of thyroid follicles with round, uniform nuclei and occasional grooves [
Figure 3:
(a and b) Hematoxylin and eosin-stained section of the metastatic brain lesion. (a) The tumor is well-demarcated from the adjacent gliotic brain parenchyma. (b) The tumor is consistent of small follicles which are composed of uniform, round nuclei without papillary thyroid carcinoma nuclear features.
The subsequent thyroid fine-needle aspiration (FNA) from the left lobe showed microfollicles and groups of crowded thyroid follicular epithelial cells with nuclear overlapping. No intranuclear grooves or inclusions were seen. Colloid was scant.
As such, these findings were diagnostic for a follicular neoplasm (Bethesda system, Category IV). Subsequently, the patient underwent total thyroidectomy. Examination of the thyroid gland revealed a 6 cm widely-invasive follicular carcinoma in the left lobe (pT3a, pN0).
Outcome and follow-up
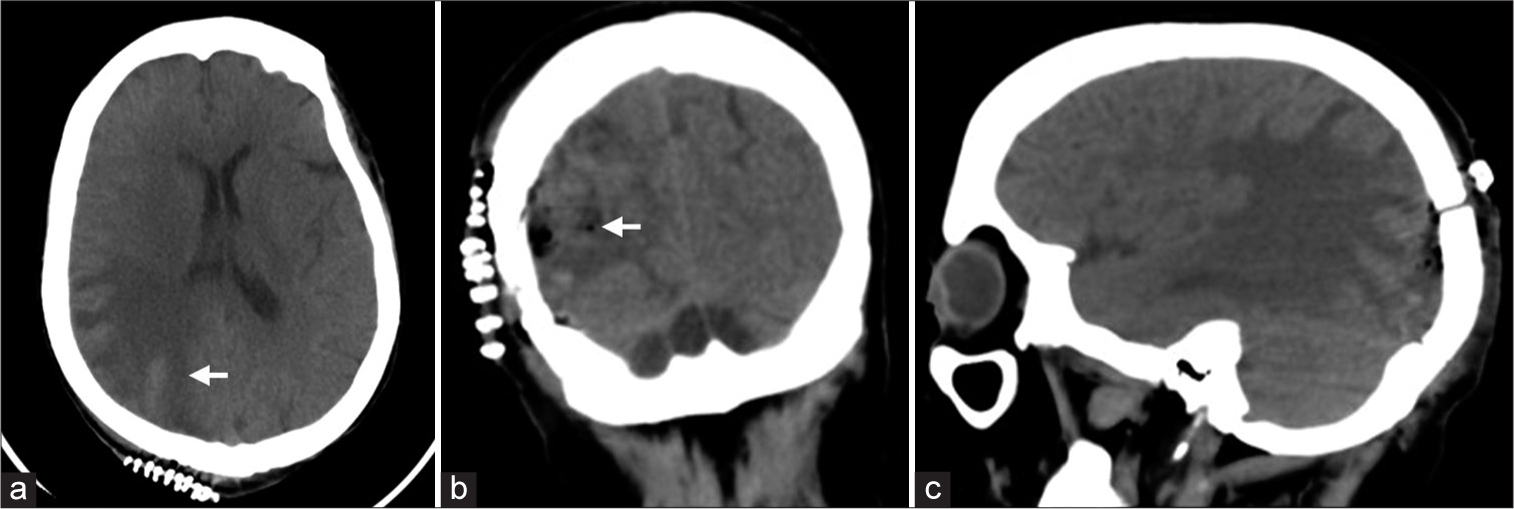
Postoperative brain CT scan demonstrated a complete resection of the lesion [
Figure 4:
(a-c) Postoperative axial, coronal, and sagittal brain CT without contrast. The images demonstrate multiple, tiny air foci within the surgical cavity, along with minimal fluid and hemorrhagic hyperdensities, representing expected postoperative changes (Arrow). There is improvement of the left-sided midline shift from 4 mm to 2 mm and partial resolution of the right parieto-occipital vasogenic edema.
A complete oncological work-up was performed to investigate the thyroid and lung masses.
The chest CT showed multiple bilateral metastatic pulmonary nodules. The findings of the positron emission tomography (PET) scan confirmed the pre-existing suspicious thyroid cancer, with metaplastic cervical lymph nodes, and bilateral pulmonary nodules. No evidence of hypermetabolic brain lesions was noted postoperatively. The iodine whole-body scan showed an iodine-refractory residual thyroid tissue on the right thyroid bed.
The patient was discharged in a stable condition with laboratory/radiological investigations and follow-up at neurosurgery, endocrine, radiotherapy, and thyroid surgery clinics.
DISCUSSION
In the present case, the diagnosis of follicular thyroid carcinoma was rendered following histopathological investigations of the occipital brain lesion and FNA of the thyroid gland. The neuroradiological imaging, including brain CT, magnetic resonance imaging, and PET, identified a solitary central nervous system lesion. The present article discusses one of the unusual sites of distant metastasis of follicular thyroid carcinoma to the brain.[
In such cases, the clinical presentation ranges from headache to focal neurological deficits, and in some cases, the diagnosis was made incidentally.[
Metastatic follicular thyroid carcinoma may exhibit various radiological features, including but not limited to; highly-enhancing masses with cystic formation, ring enhancement, varying degrees of edema, and hemorrhage.[
Considering the rarity of metastatic follicular thyroid carcinoma to the brain, the optimal management can be challenging.[
Untreated, such metastatic thyroid carcinomas tend to be associated with a short-survival rate.[
Cacho-Diaz et al. investigated approximately 400 patients with thyroid cancer.[
CONCLUSION
Follicular thyroid carcinoma may rarely metastasize to the central nervous system. A high index of suspicion is required to identify patients with thyroid cancer who initially present with neurological manifestations. Complete surgical resection of the metastatic brain lesion is safe, feasible and is associated with a prolonged overall survival.
Authors’ Contributions
Abdulaziz Alanazi: Conceptualization, Writing – Original Draft, Writing – Review and Editing. Ali Alkhaibary: Conceptualization, Project administration, Investigation – Radiological and Pathological Images, Writing – Original Draft, Writing – Review and Editing. Sami Khairy: Conceptualization, Supervision, Writing – Review and Editing. Fahd AlSufiani: Investigations – Pathological Images, Writing – Review and Edi ting. Ali H. Alassiri: Investigations – Pathological Images, Writing – Review and Editing. Ahmed Aloraidi: Supervision, Writing – Review and Editing. Ahmed Alkhani: Conceptualization, Supervision, Surgical Intervention, Writing – Review and Editing. All authors have critically reviewed and approved the final version of the manuscript.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors would like to express their gratitude to King Abdullah International Medical Research Center, Ministry of National Guard - Health Affairs, Riyadh, Saudi Arabia for approving the study. The assigned protocol number for approval is NRC21R/359/09.
References
1. Cabanillas ME, McFadden DG, Durante C. Thyroid cancer. Lancet. 2016. 388: 2783-95
2. Cacho-Diaz B, Cuevas-Ramos D, Spinola-Marono H, ReyesSoto G, Olvera-Manzanilla E, Monroy-Sosa A. Thyroid cancer brain metastases and thyroglobulin. J Endocrinol Metab. 2016. 6: 90-4
3. Chiu AC, Delpassand ES, Sherman SI. Prognosis and treatment of brain metastases in thyroid carcinoma. J Clin Endocrinol Metab. 1997. 82: 3637-42
4. Choi J, Kim JW, Keum YS, Lee IJ. The largest known survival analysis of patients with brain metastasis from thyroid cancer based on prognostic groups. PLoS One. 2016. 11: e0154739
5. De Figueiredo BH, Godbert Y, Soubeyran I, Carrat X, Lagarde P, Cazeau AL. Brain metastases from thyroid carcinoma: A retrospective study of 21 patients. Thyroid. 2014. 24: 270-6
6. Gomes-Lima CJ, Wu D, Rao SN, Punukollu S, Hritani R, Zeymo A. Brain metastases from differentiated thyroid carcinoma: Prevalence, current therapies, and outcomes. J Endocr Soc. 2018. 3: 359-71
7. Hong YW, Lin JD, Yu MC, Hsu CC, Lin YS. Outcomes and prognostic factors in thyroid cancer patients with cranial metastases: A retrospective cohort study of 4,683 patients. Int J Surg. 2018. 55: 182-7
8. Lee HS, Yoo H, Lee SH, Gwak HS, Shin SH. Clinical characteristics and follow-up of intracranial metastases from thyroid cancer. Acta Neurochir (Wien). 2015. 157: 2185-94
9. McWilliams RR, Giannini C, Hay ID, Atkinson JL, Stafford SL, Buckner JC. Management of brain metastases from thyroid carcinoma: A study of 16 pathologically confirmed cases over 25 years. Cancer. 2003. 98: 356-62
10. Patchell RA, Tibbs PA, Walsh JW, Dempsey RJ, Maruyama Y, Kryscio RJ. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990. 322: 494-500
11. Saito F, Uruno T, Shibuya H, Kitagawa W, Nagahama M, Sugino K. Prognosis after brain metastasis from differentiated thyroid carcinoma. World J Surg. 2016. 40: 574-81
12. Seib CD, Sosa JA. Evolving understanding of the epidemiology of thyroid cancer. Endocrinol Metab Clin North Am. 2019. 48: 23-35