- Department of Neurosurgery, Rhode Island Hospital, Providence, Rhode Island, United States
- Department of Pathology and Laboratory Medicine, Rhode Island Hospital, Providence, Rhode Island, United States.
Correspondence Address:
Hael Abdulrazeq, Department of Neurosurgery, Rhode Island Hospital, Providence, Rhode Island, United States.
DOI:10.25259/SNI_329_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Anna Kimata1, Hael Abdulrazeq1, Michael Kritselis2, Tori Riccelli1, Matthew Anderson1, Richard Dowd1, Ivana Dellale2, Prakash Sampath1. Metastatic cervical carcinoma to the brain masquerading as a butterfly glioma: A case report. 04-Aug-2023;14:275
How to cite this URL: Anna Kimata1, Hael Abdulrazeq1, Michael Kritselis2, Tori Riccelli1, Matthew Anderson1, Richard Dowd1, Ivana Dellale2, Prakash Sampath1. Metastatic cervical carcinoma to the brain masquerading as a butterfly glioma: A case report. 04-Aug-2023;14:275. Available from: https://surgicalneurologyint.com/surgicalint-articles/12485/
Abstract
Background: Metastatic cervical cancer to the brain is a rare occurrence, representing approximately 1.5% of metastatic cases. We report a rare presentation of cervical cancer with brain metastasis to the corpus callosum. The patient was initially suspected to have a primary glioma but was diagnosed with a metastatic cervical carcinoma lesion through both stereotactic and then opens biopsy.
Case Description: A 53-year-old female, with Stage III adenosquamous cervical carcinoma, presented with a large heterogeneously enhancing mass in the corpus callosum body with extension in the cingulate gyrus concerning for glioma. A stereotactic biopsy revealed hypercellular and gliotic brain tissue, while an open biopsy showed an epithelioid neoplasm consistent with metastatic cervical adenosquamous carcinoma. The patient underwent a craniotomy and recovered well and was discharged in stable condition.
Conclusion: Brain metastases from cervical cancer are uncommon. We present a rare case of metastatic cervical carcinoma which appeared on imaging to mimic a butterfly glioma. The patient’s history and histopathological examination were essential in determining the correct diagnosis and receiving timely treatment.
Keywords: Metastatic cervical carcinoma, Neuro oncology, Neuropathology
INTRODUCTION
Metastatic brain tumors can originate from a variety of primary cancers. Tumors of the lung, breast, and skin are the most frequent primary sites. Cervical cancer represents the fourth most common malignancy impacting female-identifying patients, with a global incidence of almost 500,000 new cases and 300,000 deaths/year.[
CASE DESCRIPTION
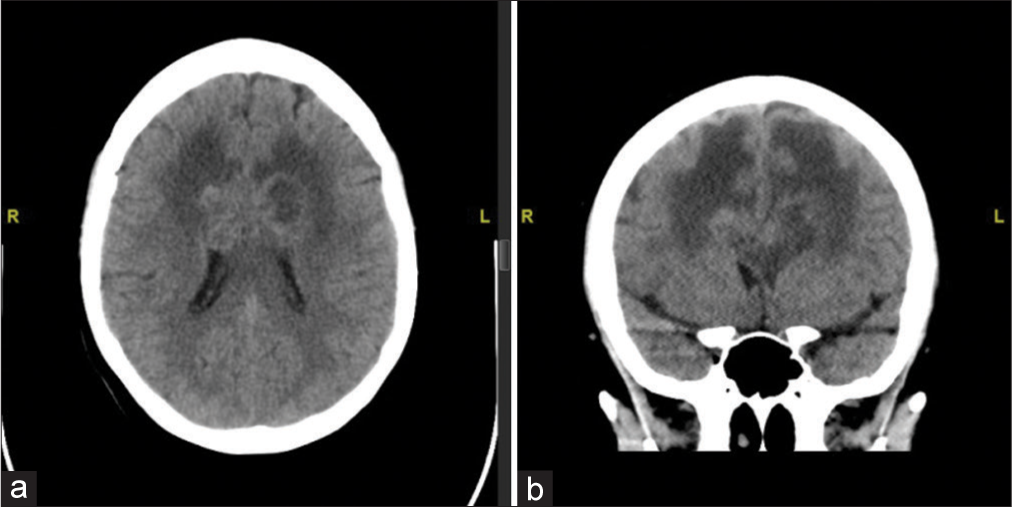
A 53-year-old female with medical history of Stage III adenosquamous cervical carcinoma which was diagnosed 20 months before presentation, with pulmonary involvement on Pembrolizumab, generalized anxiety disorder, and depression, presents to the emergency room with behavioral changes, difficulty with writing, and leg shaking. On examination, the patient had normal and stable vital signs and was neurologically intact with no deficits. A computed tomography (CT) scan was obtained which demonstrated vasogenic edema the bilateral frontal lobes with involvement of the corpus callosum, and evidence of an ill-defined mass centered in the corpus callosum above the anterior horns of the lateral ventricles [
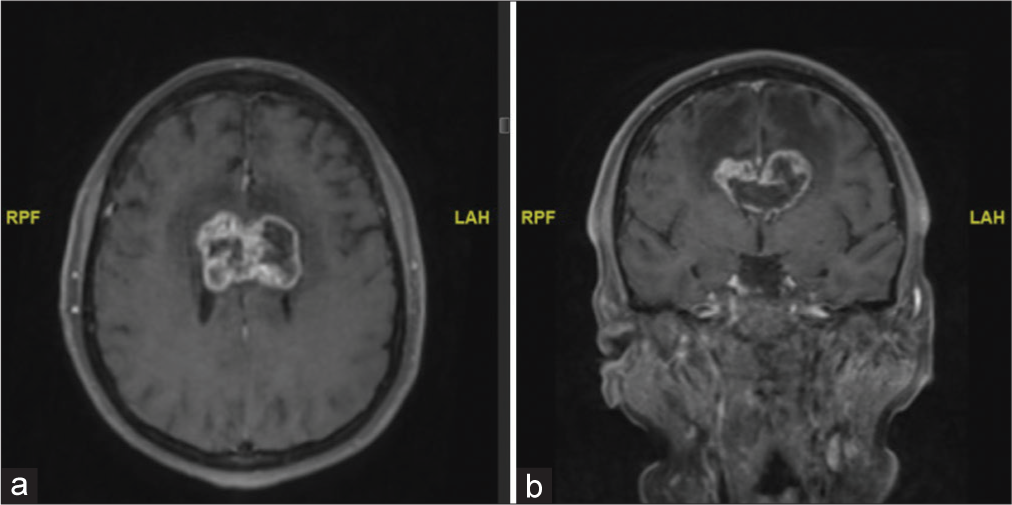
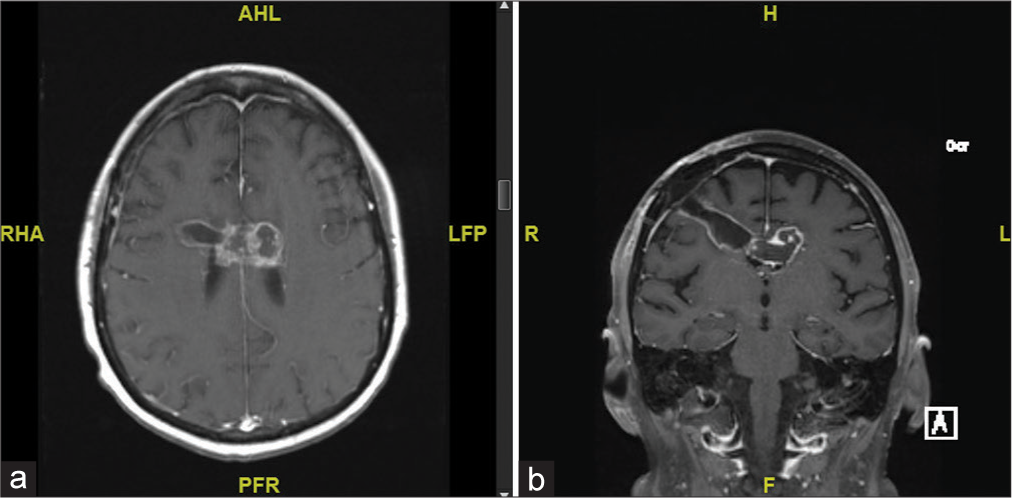
The patient was given intravenous dexamethasone and admitted for further work up and neurosurgical intervention. A magnetic resonance image (MRI) of the brain with contrast was obtained which showed a large heterogeneously enhancing mass in the corpus callosum body with extension in the cingulate gyrus and involving the lateral ventricles. The mass included some areas of diffusion restriction. There was extensive bifrontal vasogenic edema with no evidence of hemorrhage [
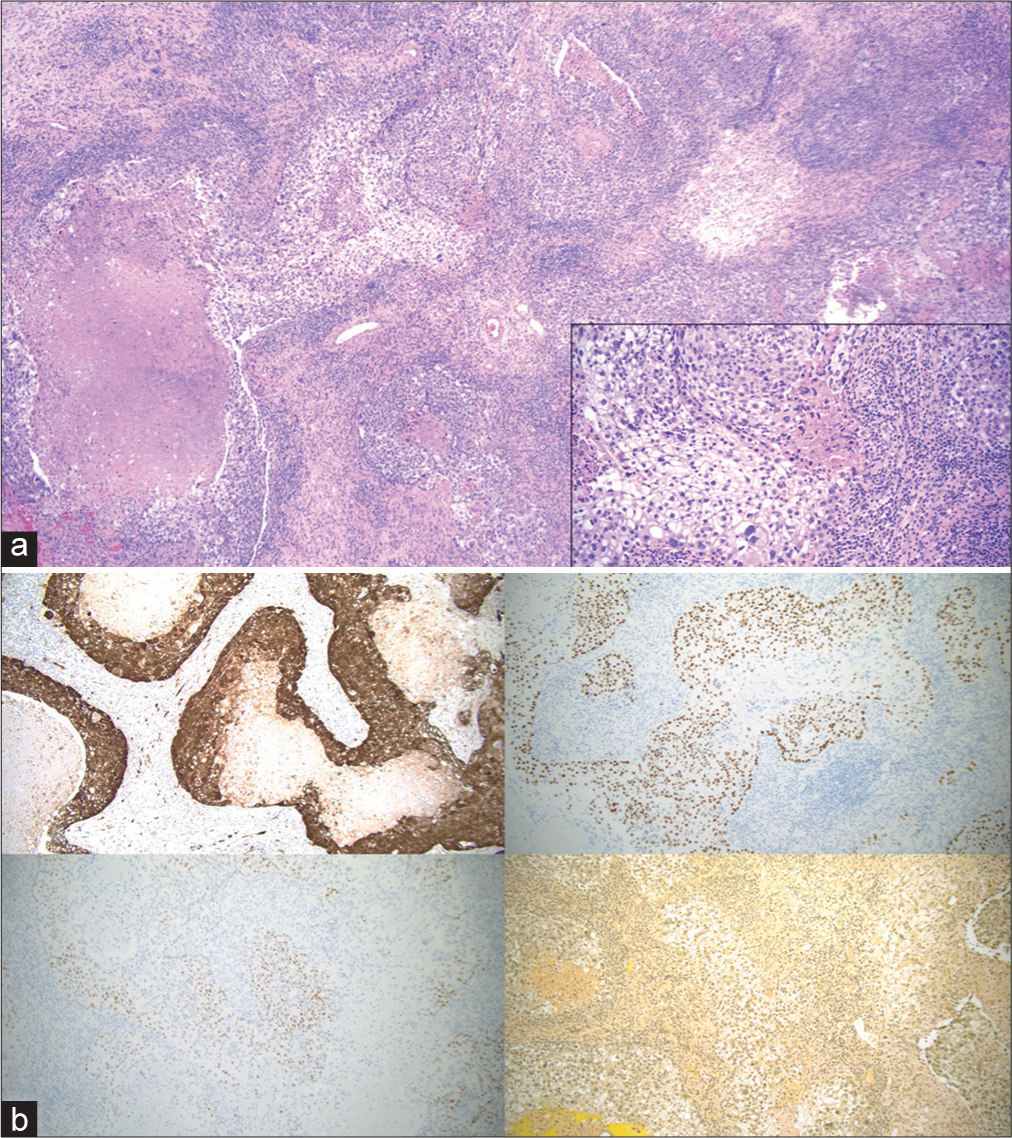
Due to the appearance of a typical butterfly glioma on imaging, the patient underwent a stereotactic biopsy to obtain a specimen for histopathological analysis. The stereotactic biopsy was obtained, and on frozen section was noted to contain hypercellular and gliotic brain tissue. Due to sampling and small specimen size, the treatment team proceeded with a right-sided craniotomy for open biopsy and partial resection to obtain more specimens for analysis. The specimen from the open biopsy demonstrated an epithelioid neoplasm in a background of necrosis, with tumor cells showing pleomorphism, nuclear atypia, glycogenization, and abundant mitotic figures. Immunohistochemical staining was positive for estrogen, p16 Protein, and P40. The histopathology and immunohistochemistry were consistent with a metastatic lesion from the patient’s known cervical adenosquamous carcinoma [
Figure 3:
(a) Microscopic examination reveals a hypercellular epithelioid neoplasm with abundant mitotic activity in a background of necrosis. Focal areas of tumor cells demonstrate clear cytoplasm with distinct cell borders, consistent with glycogenation (inset). (b) Immunohistochemical staining reveals the tumor cells are positive for p16 (upper left), p40 (upper right), and estrogen receptor (lower left). The tumor cells are negative for PAX8 and progesterone receptor. Histochemical staining with mucicarmine is negative for any intracellular mucin (lower right). The overall findings are consistent with metastatic HPV-dependent squamous cell carcinoma. (a) Hematoxylin and eosin, ×4 (inset: ×20). (b) Hematoxylin and eosin, ×10.
Figure 4:
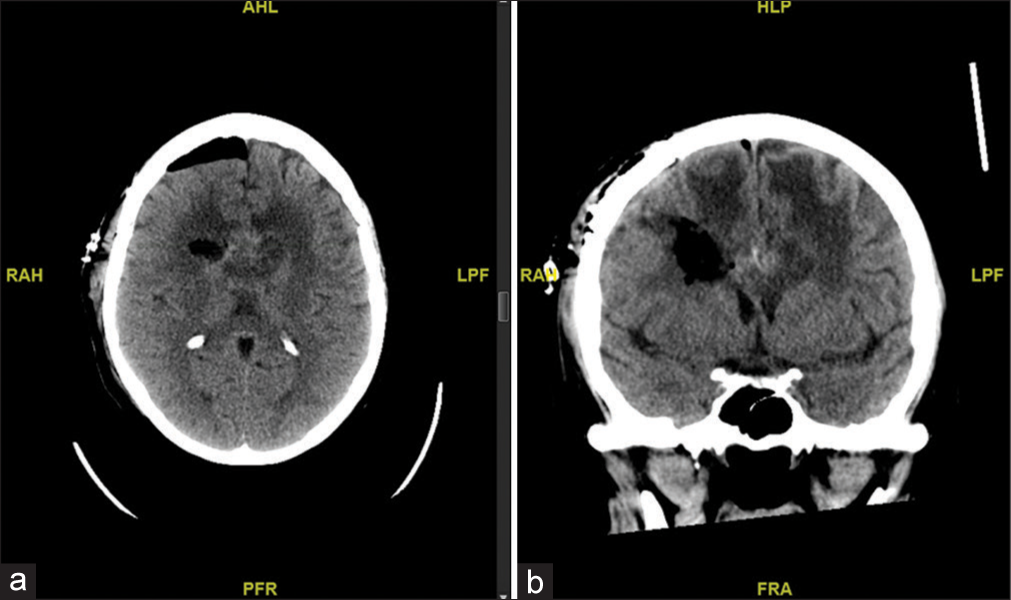
Axial (a) and coronal (b) sequences of computed tomography brain without contrast status post right frontal craniotomy demonstrating expected postoperative changes of open biopsy and partial resection of callosal mass. AHL: Anterior. RAH: Right. LPF: Left. PFR: Posterior. HLP: Superior. FRA: Inferior.
Figure 5:
Axial (a) and coronal (b) sequences of follow-up magnetic resonance image brain with contrast 3-month postoperatively demonstrating interval decreased size of enhancing mass centered within the corpus callosum body and thin marginal enhancement along the biopsy tract, favored to represent postsurgical change. RHA: Right. LFP: Left. AHL: Anterior. PFR: Posterior.
DISCUSSION
On initial presentation, the patient above was presumed to likely have a primary glial neoplasm due to the appearance of butterfly glioma on imaging, despite her history of metastatic cervical adenocarcinoma. Lymphoma was also high on the differential diagnosis list, but given the suspicion for glioma, lumbar puncture was deferred, and the team proceeded with biopsy for tissue diagnosis. Corpus callosum lesions which have the classic “butterfly” appearance develop due to spread of pathology along the white matter tracts. Given the dense myelin composition of these myelin tracts, growth of tumors in this region indicates an aggressive pathology. In addition, the rich myelinated axon content makes this region susceptible to demyelinating processes such as multiple sclerosis and progressive multifocal leukoencephalopathy.[
Cervical cancer is a major global health and financial concern, with one of the highest annual incidences worldwide and poor prognosis.[
Recent work has shown that patients with brain metastases have overall similar survival to those with other single site organ metastases and metastases to multiple sites, indicating that timely management of these cases can result in comparable outcomes for these patients.[
Although brain metastases from cervical cancer are uncommon, there are recognizable patterns of symptoms that occur in patients with metastatic lesions, which include elevated intracranial pressure, headache, nausea, vomiting, seizures, and extremity weakness.[
CONCLUSION
We present a case of metastatic cervical cancer to the corpus callosum with a radiographic appearance suspicious for “butterfly” glioma. The combination of both the location of this metastatic lesion as well as its primary cancer makes this case exceedingly rare and emphasizes the importance of maintaining an encompassing differential when diagnosing newly identified brain lesions. It is therefore critical to obtain and consider a full medical and oncologic history of the patient, beyond what the imaging data may suggest.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Agrawal A, Kumar A, Sinha AK, Kumar M, Pandey SR, Khaniya S. Intracranial metastases from carcinoma of the cervix. Singapore Med J. 2007. 48: e154-6
2. Amita M, Sudeep G, Rekha W, Yogesh K, Hemant T. Brain metastasis from cervical carcinoma--a case report. MedGenMed. 2005. 7: 26
3. Bourekas EC, Varakis K, Bruns D, Christoforidis GA, Baujan M, Slone HW. Lesions of the corpus callosum: MR imaging and differential considerations in adults and children. AJR Am J Roentgenol. 2002. 179: 251-7
4. Brito Rangel J, Giglio AG, Cardozo CL, Bergmann A, Thuler LC. Prognostic factors for brain metastasis in women presenting cervical cancer. J Neurooncol. 2022. 159: 469-77
5. Chura JC, Shukla K, Argenta PA. Brain metastasis from cervical carcinoma. Int J Gynecol Cancer. 2007. 17: 141-6
6. Cronin KA, Ries LA, Edwards BK. The surveillance, epidemiology, and end results (SEER) program of the national cancer institute. Cancer. 2014. 120: 3755-7
7. Divine LM, Kizer NT, Hagemann AR, Pittman ME, Chen L, Powell MA. Clinicopathologic characteristics and survival of patients with gynecologic malignancies metastatic to the brain. Gynecol Oncol. 2016. 142: 76-82
8. Fetcko K, Gondim DD, Bonnin JM, Dey M. Cervical cancer metastasis to the brain: A case report and review of literature. Surg Neurol Int. 2017. 8: 181
9. Fink KR, Fink JR. Imaging of brain metastases. Surg Neurol Int. 2013. 4: S209-19
10. Franco EL, Schlecht NF, Saslow D. The epidemiology of cervical cancer. Cancer J. 2003. 9: 348-59
11. Garber ST, Khoury L, Bell D, Schomer DF, Janku F, McCutcheon IE. Metastatic adenoid cystic carcinoma mimicking butterfly glioblastoma: A rare presentation in the splenium of the corpus callosum. World Neurosurg. 2016. 95: 621.e13-19
12. Li H, Wu X, Cheng X. Advances in diagnosis and treatment of metastatic cervical cancer. J Gynecol Oncol. 2016. 27: e43
13. Pyeon SY, Park JY, Ulak R, Seol HJ, Lee JM. Isolated brain metastasis from uterine cervical cancer: A case report and review of literature. Eur J Gynaecol Oncol. 2015. 36: 602-4
14. Sperduto PW, Berkey B, Gaspar LE, Mehta M, Curran W. A new prognostic index and comparison to three other indices for patients with brain metastases: An analysis of 1,960 patients in the RTOG database. Int J Radiat Oncol Biol Phys. 2008. 70: 510-4
15. Takayanagi A, Florence TJ, Hariri OR, Armstrong A, Yazdian P, Sumida A. Brain metastases from cervical cancer reduce longevity independent of overall tumor burden. Surg Neurol Int. 2019. 10: 176
16. Ucmakli A. The pathological significance of corpus callosum involvement in brain scans. J Nucl Med. 1972. 13: 510-6
17. Zhou S, Peng F. Patterns of metastases in cervical cancer: A population-based study. Int J Clin Exp Pathol. 2020. 13: 1615-23