Metastatic choroid plexus papilloma presenting as a sellar mass: A case report and literature review
- Department of Neurosurgery, State University of New York (SUNY) Upstate Medical University, Syracuse, New York, United States.
Correspondence Address:
Brandon Michael Wilkinson, Department of Neurosurgery, State University of New York (SUNY) Upstate Medical University, Syracuse, New York, United States.
DOI:10.25259/SNI_847_2023
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Brandon Michael Wilkinson, Michael A. Duncan, Dan Y. Draytsel, Harish Babu. Metastatic choroid plexus papilloma presenting as a sellar mass: A case report and literature review. 19-Apr-2024;15:140
How to cite this URL: Brandon Michael Wilkinson, Michael A. Duncan, Dan Y. Draytsel, Harish Babu. Metastatic choroid plexus papilloma presenting as a sellar mass: A case report and literature review. 19-Apr-2024;15:140. Available from: https://surgicalneurologyint.com/surgicalint-articles/12860/
Abstract
Background: Choroid plexus papillomas (CPPs) are rare neoplasms arising from choroid plexus epithelium representing de novo formation in extraventricular sites is rare.
Case Description: A 57-year-old female with a history of a 4th ventricular CPP status post resection in 2004 and 2018 with subsequent gamma knife therapy in 2019 presented with increased thirst and urination. Since her initial surgery, she has experienced significant gait imbalance, diplopia, dysphagia, and right-sided hemiparesis and hemisensory loss. Magnetic resonance imaging revealed a new 1.5 × 1.8 cm suprasellar lesion. She underwent a left supraorbital craniotomy for tumor resection, with pathology revealing metastatic World Health Organization grade II CPP.
Conclusion: Extraventricular manifestation of CPP is rare. De novo or metastatic involvement of the sella has seldom been reported. Treatment should target gross total surgical resection. Adjuvant chemotherapy and radiation may be useful in higher-grade lesions.
Keywords: Choroid plexus papilloma, Metastatic, Sella, Supraorbital craniotomy
INTRODUCTION
Choroid plexus papillomas (CPPs) are rare neoplasms arising from choroid plexus epithelium and represent <1% of all intracranial tumors.[
According to the World Health Organization (WHO) classification, choroid plexus tumors are classified as papillomas (grade I), atypical tumors (grade II), and carcinomas (CPC; grade III) based on the number of mitotic figures per high-power field on histologic examination. Papillomas are typically soft, friable masses with high vascularity.[
Less commonly, CPP may invade surrounding parenchyma or disseminate throughout the central nervous system (CNS). Cases of leptomeningeal spread and the formation of numerous cystic lesions in both the brain and spine have been reported.[
Here, we present a 57-year-old patient with prior 4th ventricular CPP with a solid distant metastasis to the sella, treated with supraorbital craniotomy for resection.
CASE PRESENTATION
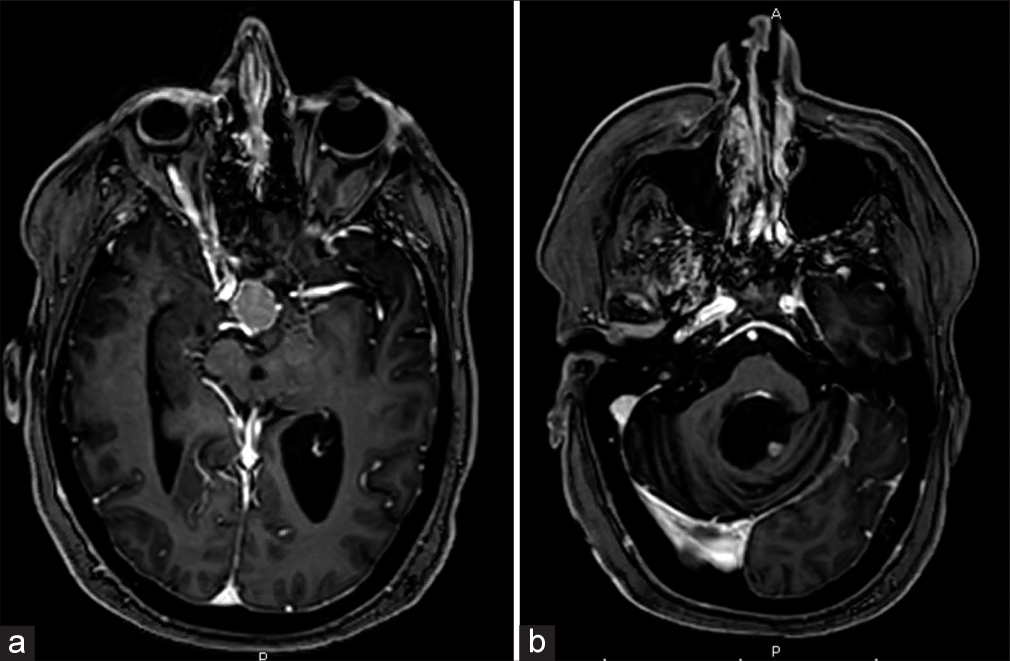
A 57-year-old female with a history of a 4th ventricular CPP presented with increased thirst and urination. She initially underwent suboccipital craniotomy for tumor resection in 2004 and again in 2018 for tumor recurrence. She received subsequent Gamma Knife radiotherapy in 2019. Pathology from both resections revealed atypical CPP, WHO grade II. Since her initial surgery, she has experienced significant gait imbalance, diplopia, dysphagia, and right-sided hemiparesis and hemisensory loss. Surveillance magnetic resonance imaging (MRI) was done, showing an enhancing 1.5 × 1.8 cm suprasellar lesion, as well as a stable residual 5 × 5 mm lesion in the left posterolateral margin of the 4th ventricle [
Figure 1:
(a) Preoperative axial T1-weighted magnetic resonance imaging (MRI) brain with contrast showing a 1.5 × 1.8 cm sellar mass. (b) Preoperative axial T1-weighted MRI brain with contrast showing a small 5 × 5 mm enhancing nodule in the left posterolateral margin of the 4th ventricle, representing residual choroid plexus papilloma.
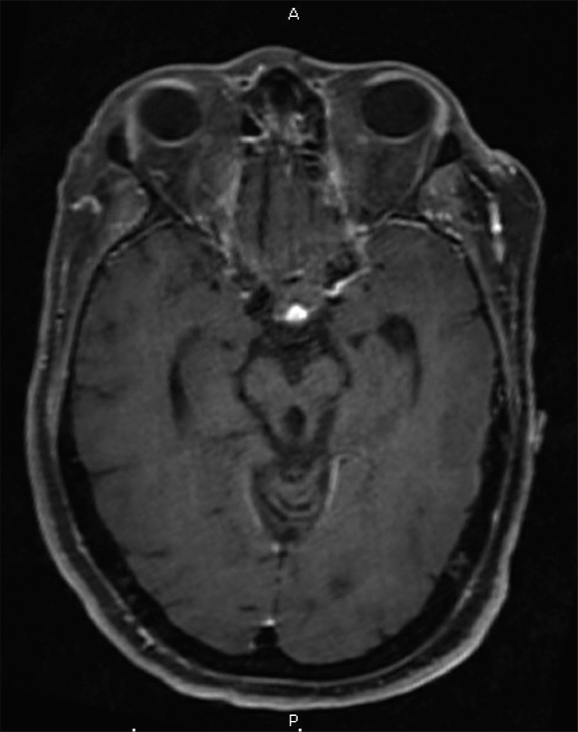
The patient was treated with a left supraorbital craniotomy for tumor resection. Given her baseline functional status, we felt that an eyebrow incision provided the least invasive approach to access the tumor. A craniotomy was performed on the left side due to her existing right-sided deficits. We decided against an endoscopic transnasal transsphenoidal approach, given the patient’s obesity and associated increased risk for CSF leak. We additionally felt an endonasal approach and potential complications may exacerbate the patient’s baseline dysphagia. During resection, the firm lesion was densely adherent to the hypothalamic surface. Meticulous dissection with microinstruments allowed a tissue plane to be developed, and tumor resection continued toward the sellar floor. Preservation of functioning pituitary tissue was attempted; however, the normal-appearing pituitary gland was not visualized throughout tumor dissection and debulking. A small tumor was residual and densely adherent to the optic apparatus and was left behind to allow vision preservation. Closure was completed in standard fashion using a pericranial graft for watertight dural repair. Similarly to her prior surgeries, pathology revealed WHO II atypical CPP, suggesting metastasis. She developed panhypopituitarism treated with a regimen of desmopressin, levothyroxine, and hydrocortisone. She had a prolonged hospital course complicated by worsened dysphagia and altered mental status attributed to hypoxia secondary to atelectasis. Her respiratory status gradually improved with positive pressure ventilation through BiPAP, and her mental status returned to her preoperative baseline. Her worsened that dysphagia, however, did persist and ultimately required gastrostomy tube placement. Postoperative imaging demonstrated a small residual lesion within the sella [
DISCUSSION
Although rare, there have been several documented cases of CPP with distant lesions throughout the neuroaxis. Metastatic lesions may present as solid-enhancing lesions, “drop metastases” in the spine, or leptomeningeal seeding.[
Numerous widespread cystic lesions have also been described. Johnson et al. reviewed a series of eight such patients.[
Given the reported cases of disseminated CPP, Mazur-Hart et al. proposed a new classification system describing the spectrum of disease.[
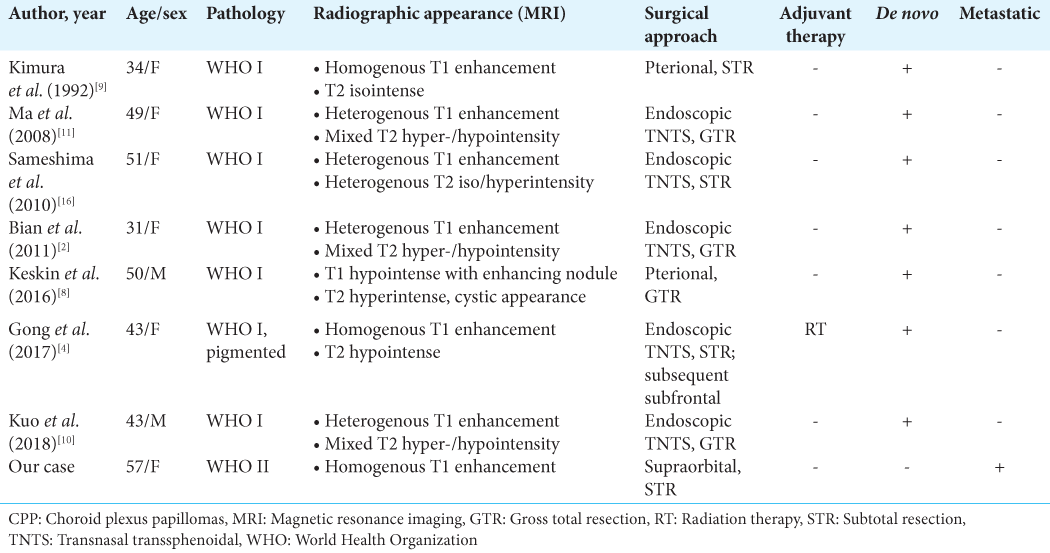
Solid lesions involving the sellar region appear to be exceptionally rare. We identified eight publications describing eight patients with sellar CPP, including our own [
While the mainstay of treatment involves gross total resection, the role of adjuvant therapies is less well-established. Chemotherapy, particularly in cases of CPC, has been reported to increase overall survival.[
The findings in our case highlight the importance of recognizing potential metastasis in CPP, including new lesions that may arise in extraventricular locations. Sellar lesions may mimic typical adenomas or craniopharyngioma; however, in patients with a history of known CPP, metastatic disease should remain in the differential. Multiple lesions may arise regardless of pathologic grade. Surveillance imaging remains essential, and full neuroaxis imaging should be considered to identify potential distant spread.
CONCLUSION
Extraventricular manifestation of CPP is rare. De novo or metastatic involvement of the sella has seldom been reported. Treatment should involve gross total surgical resection to prevent recurrence. Adjuvant chemotherapy and radiation may be appropriate in higher-grade lesions.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Abdulkader MM, Mansour NH, Van Gompel JJ, Bosh GA, Dropcho EJ, Bonnin JM. Disseminated choroid plexus papillomas in adults: A case series and review of the literature. J Clin Neurosci. 2016. 32: 148-54
2. Bian LG, Sun QF, Wu HC, Jiang H, Sun YH, Shen JK. Primary choroid plexus papilloma in the pituitary fossa: Case report and literature review. Acta Neurochir (Wien). 2011. 153: 851-7
3. Demir MK, Özdamarlar U, Yılmaz B, Akakın A, Kılıc T. Magnetic resonance imaging of unusual neoplasms related to foramen of luschka: A review for differential diagnosis. Indian J Radiol Imaging. 2022. 32: 71-80
4. Gong X, Liu C, Zhang L, Li Z, Bartley CM, Liu Z. A primary pigmented choroid plexus papilloma located within the sella turcica: Case report and literature review. World Neurosurg. 2017. 105: 1039.e13-8
5. Jeibmann A, Hasselblatt M, Gerss J, Wrede B, Egensperger R, Beschorner R. Prognostic implications of atypical histologic features in choroid plexus papilloma. J Neuropathol Exp Neurol. 2006. 65: 1069-73
6. Johnson GW, Mian AY, Dahiya S, Rich KM, Chicoine MR, Limbrick DD. Cystic dissemination of choroid plexus papilloma: Illustrative cases. J Neurosurg Case Lessons. 2022. 4: CASE22360
7. Kabashi A, Ahmetgjekaj I. Choroid plexus papilloma-case presentation. Curr Health Sci J. 2021. 47: 310-3
8. Keskin F, Erdi F, Kaya B, Toy H. Sellar-suprasellar extraventricular choroid plexus papilloma: A case report and review of the literature. J Korean Neurosurg Soc. 2016. 59: 58-61
9. Kimura M, Takayasu M, Suzuki Y, Negoro M, Nagasaka T, Nakashima N. Primary choroid plexus papilloma located in the suprasellar region: Case report. Neurosurgery. 1992. 31: 563-6
10. Kuo CH, Yen YS, Tu TH, Wu JC, Huang WC, Cheng H. Primary choroid plexus papilloma over sellar region mimicking with craniopharyngioma: A case report and literature review. Cureus. 2018. 10: e2849
11. Ma YH, Ye K, Zhan RY, Wang LJ. Primary choroid plexus papilloma of the sellar region. J Neurooncol. 2008. 88: 51-5
12. Mazur-Hart DJ, Yaghi NK, Larson EW, Pang BW, Woltjer RL, Pettersson DR. Rare case of pediatric disseminated choroid plexus papilloma: Literature review and call for reclassification. Pediatr Neurosurg. 2022. 57: 348-57
13. Ortega-Martínez M, Cabezudo-Artero JM, FernándezPortales I, Pimentel JJ, Gómez de Tejada R. Diffuse leptomeningeal seeding from benign choroid plexus papilloma. Acta Neurochir (Wien). 2007. 149: 1229-37
14. Pencalet P, Sainte-Rose C, Lellouch-Tubiana A, Kalifa C, Brunelle F, Sgouros S. Papillomas and carcinomas of the choroid plexus in children. J Neurosurg. 1998. 88: 521-8
15. Ruggeri L, Alberio N, Alessandrello R, Cinquemani G, Gambadoro C, Lipani R. Rapid malignant progression of an intraparenchymal choroid plexus papillomas. Surg Neurol Int. 2018. 9: 131
16. Sameshima T, Tanikawa R, Sugimura T, Izumi N, Seki T, Maeda T. Choroid plexus papilloma originating in the sella turcica-case report. Neurol Med Chir (Tokyo). 2010. 50: 144-6
17. Sharma R, Rout D, Gupta AK, Radhakrishnan VV. Choroid plexus papillomas. Br J Neurosurg. 1994. 8: 169-77
18. Tavallaii A, Keykhosravi E, Rezaee H, Kianbakht C. Role of available adjuvant therapies following surgical resection of atypical choroid plexus papilloma-a systematic review and pooled analysis. Neurooncol Adv. 2020. 2: vdaa139
19. Wolff JE, Van Gool SW, Kutluk T, Diez B, Kebudi R, Timmermann B. Final results of the choroid plexus tumor study CPT-SIOP-2000. J Neurooncol. 2022. 156: 599-613