- Department of Orthopedics, Sancheti Institute of Orthopedics and Rehabilitation, Pune, Maharashtra, India,
- Department of Orthopedics, University of Dundee, Dundee, Scotland, United Kingdom.
Correspondence Address:
Amogh Zawar
Department of Orthopedics, Sancheti Institute of Orthopedics and Rehabilitation, Pune, Maharashtra, India,
DOI:10.25259/SNI_343_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Shailesh Hadgaonkar, Amogh Zawar, Anoop Patel, Ajay Kothari, Ashok Shyam, Parag Sancheti. Metastatic small cell neuroendocrine tumor with spinal cord compression – Understanding a rare entity. 11-Jul-2020;11:185
How to cite this URL: Shailesh Hadgaonkar, Amogh Zawar, Anoop Patel, Ajay Kothari, Ashok Shyam, Parag Sancheti. Metastatic small cell neuroendocrine tumor with spinal cord compression – Understanding a rare entity. 11-Jul-2020;11:185. Available from: https://surgicalneurologyint.com/surgicalint-articles/10127/
Abstract
Background: Metastatic spinal cord compression with carcinoid tumor as primary is a rare entity with its own diagnostic dilemmas and surgical challenges. Most of these neuroendocrine tumors arise from the gastrointestinal tract or lungs with metastasis to spine in
Case Description: The authors describe a case report on similar lines of a middle aged gentleman presenting with low back pain and weakness in both lower limbs which on further investigations revealed a pathological fracture causing spinal cord compression due to metastasis from small cell carcinoma in the lungs, managed with surgical intervention, and subsequently with radiotherapy.
Conclusion: Secondary metastatic deposits in the lumbar vertebrae due to carcinoid tumors in the lungs are a rare entity and can be difficult to diagnose and manage further. However, it should be included in the list of differential diagnosis. The case report emphasizes on using investigative modalities such as PET-CT scan to aid an early diagnosis and plan further treatment plan as early as possible to offer a better quality of life to the patients.
Keywords: Carcinoid tumors, Lumbar spine, Metastasis, Spinal cord compression
INTRODUCTION
Carcinoid tumors are neoplasms arising from neuroendocrine enterochromaffin cells with an overall incidence of 1–2 in 100,000.[
CASE REPORT
A 49-year-old male presented with 2 months of low back pain (VAS score 8), and the recent onset of paraparesis (ODI score 80). On examination, he had bilateral quadriceps weakness (3/5) and a bilateral foot drop (0/5), diminished patellar and Achilles responses (1+), and decreased pain appreciation in the L3– L5 distributions.
Diagnostic studies
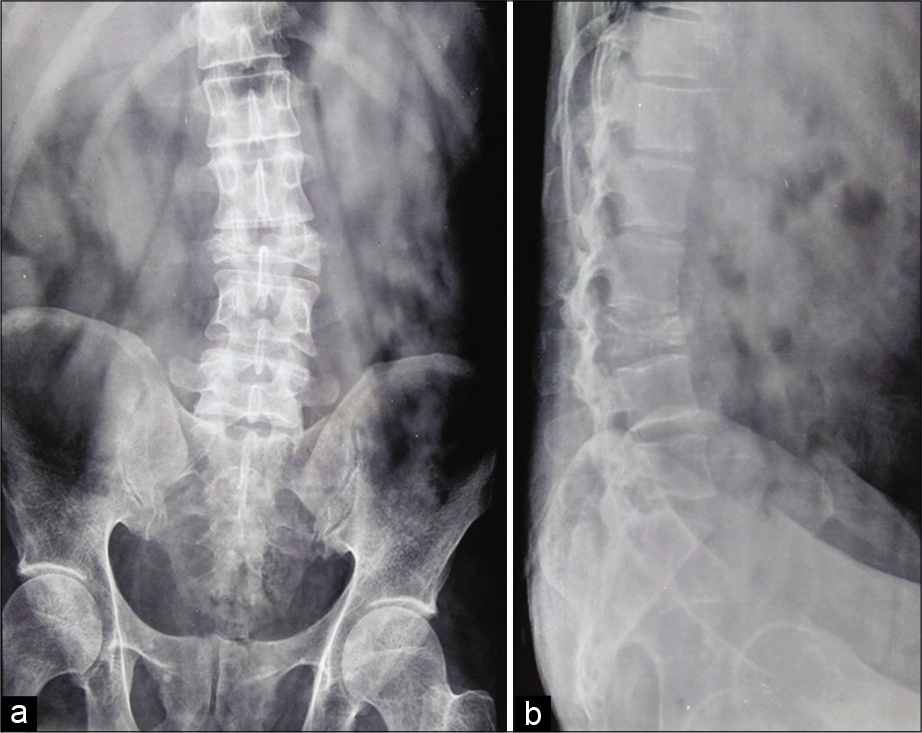
The plain lumbosacral radiographs showed a L3 compression fracture (50% loss of height) [
Surgery
The patient underwent a L3 corpectomy with mesh cage placement, followed by a L1–L5 laminectomy with pedicle screw/rod fixation. On removing the L2–L4 lamina, a grayish-white mass was observed compressing the spinal cord [
Pathology
Immunohistochemistry (IHC) studies were consistent with metastatic deposits of small cell (neuroendocrine) carcinoma. They were positive for cytokeratin, CD56, and synaptophysin markers with MIB-1 proliferation index of 70%, all consistent with metastatic carcinoid disease [
Adjunctive therapy
Postoperatively, with the diagnosis of an original primary lung carcinoid tumor with multilevel spinal metastases, the patient underwent adjunctive radiation therapy to the lumbar spine and pelvis (e.g., using six MV photons, using a total dose of 20Gy in five fractions).
Follow-up
Six weeks following surgery, the patient was able to ambulate with a walker [
DISCUSSION
Frequency, clinical findings, and diagnostic delays
Carcinoid tumors are slow-growing malignancies; bony metastases to the spine occur in about 2% of patients and most commonly involve the thoracic vertebrae (40%).[
X-ray/bone scan/PET-CT findings
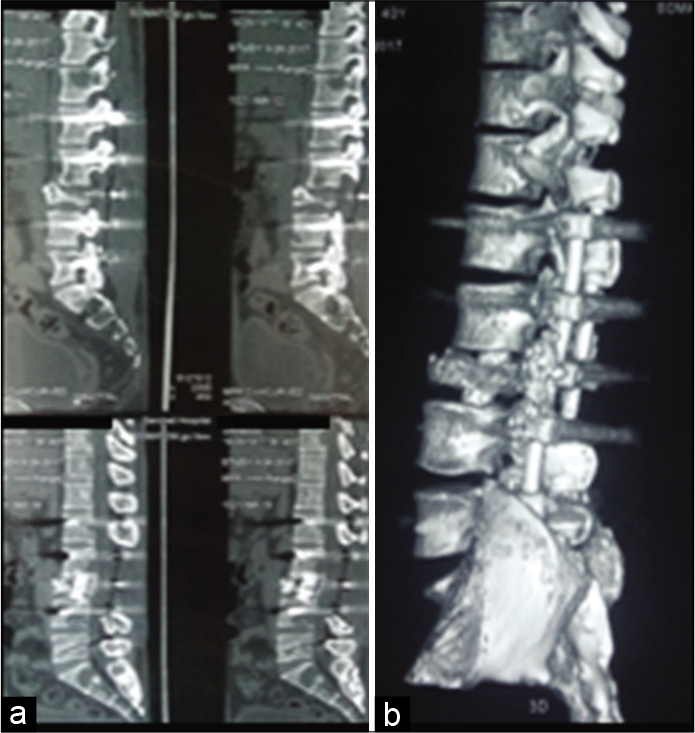
Plain radiographs and MRI, along with bone scintigraphy, octreotide scintigraphy, and PET-CT facilitate the staging of metastatic carcinoid to the spine and pelvis. Although most skeletal carcinoid metastases in the literature were osteoblastic,[
Patient survival and adjuvant radiotherapy
Patient survival varies from few weeks to up to 2 years. Radiotherapy is an important palliative treatment.[
CONCLUSION
MR and PET-CT studies best document carcinoid metastases to the spine. Avoiding diagnostic delays and opting for early spinal/neural decompression and/or appropriate utilization of adjuvant radiation therapy achieve the best outcomes.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Arnold PM, Floyd HE, Anderson KK, Newell KL. Surgical management of carcinoid tumors metastatic to the spine: Report of three cases. Clin Neurol Neurosurg. 2010. 112: 443-5
2. Bailey D, Robustelli B, Mayer J, Andrews J. Metastatic neuroendocrine tumor presenting as spinal cord compression--a case report and brief comment. Conn Med. 2001. 65: 585-6
3. Blondet E, Dulou R, Camparo P, Pernot P. Lumbar intradural metastasis of a primary carcinoid tumor of the lung: Case illustration. J Neurosurg Spine. 2005. 2: 231-1
4. Hori T, Yasuda T, Suzuki K, Kanamori M, Kimura T. Skeletal metastasis of carcinoid tumors: Two case reports and review of the literature. Oncol Lett. 2012. 3: 1105-8
5. Meijer WG, van der Veer E, Jager PL, van der Jagt EJ, Piers BA, Kema IP. Bone metastases in carcinoid tumors: Clinical features, imaging characteristics, and markers of bone metabolism. J Nucl Med. 2003. 44: 184-91
6. Scott S, Antwi-Yeboah Y, Bucur S. Metastatic carcinoid tumor with spinal cord compression. J Surg Case Rep. 2012. 2012: 5-
7. Tanabe M, Akatsuka K, Umeda S, Shomori K, Taniura S, Okamoto H. Metastasis of carcinoid to the arch of the axis in a multiple endocrine neoplasia patient: A case report. Spine J. 2008. 8: 841-4
8. Van Loon K, Zhang L, Keiser J, Carrasco C, Glass K, Ramirez MT. Bone metastases and skeletal-related events from neuroendocrine tumors. Endocr Conn. 2015. 4: 9-17