- Department of Neurosurgery, LINT, Facultad de Medicina, Universidad Nacional de Tucumán,
- Department of Neurological Surgery, Hospital Padilla, Tucumán, Argentina
- Department of Neurological Surgery, Hospital San Fernando, Argentina
- Laboratory of Microsurgical Neuroanatomy, Second Chair of Gross Anatomy, School of Medicine, University of Buenos Aires, Buenos Aires, Argentina,
- Department of Neurosurgery, Medical University of South Carolina, Charleston, South Carolina, USA,
- Neurosurgery Unit, Department of Clinical-Surgical, Diagnostic and Pediatric Sciences, University of Pavia, Italy.
- Neurosurgery Unit, Department of Surgical Sciences, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy.
Correspondence Address:
Sabino Luzzi, Department of Neurosurgery, University of Pavia, Polo Didattico “Cesare Brusotti”, Pavia, Italy.
DOI:10.25259/SNI_68_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Alvaro Campero1,2, Matías Baldoncini3,4, Jaime Martinez5, Juan F. Villalonga1,2, Alice Giotta Lucifero6, Sabino Luzzi6,7. Microneurosurgical management of aneurysms of the A1 segment of the anterior cerebral artery: Anatomy and surgical technique. 22-Jul-2022;13:310
How to cite this URL: Alvaro Campero1,2, Matías Baldoncini3,4, Jaime Martinez5, Juan F. Villalonga1,2, Alice Giotta Lucifero6, Sabino Luzzi6,7. Microneurosurgical management of aneurysms of the A1 segment of the anterior cerebral artery: Anatomy and surgical technique. 22-Jul-2022;13:310. Available from: https://surgicalneurologyint.com/surgicalint-articles/11740/
Abstract
Background: Aneurysms of the A1 segment of the anterior cerebral artery (ACA) are rare and have characteristics differentiating them from other intracranial aneurysms. Their microsurgical management is challenging and requires different strategies. In this article, we review the surgical anatomy of the A1 segment of the ACA with cadaveric dissections and describe the microsurgical management of complex A1 aneurysms with illustrative cases.
Methods: A right pterional craniotomy and Sylvian dissection were performed on a formalin-fixed and silicone-injected cadaver head to depict the key anatomic structures and surgical corridors for microsurgical clipping of A1 segment aneurysms. The microneurosurgical management of ruptured and unruptured aneurysms of the A1 segment of the ACA is described with case illustrations.
Results: The A1 segment of the ACA can be subdivided into proximal, middle, and distal subsegments, the former having abundant perforating branches. Both patients treated with microsurgical clipping had excellent and durable outcomes and postoperative cerebral angiograms showed complete aneurysm occlusion.
Conclusion: Small A1 aneurysms may require early treatment as their rupture risk appears to be higher. A1 aneurysms are usually embedded in perforators, especially those arising from the proximal A1 subsegment, and require careful distal to proximal microdissection and strategic placement of the aneurysm clip blades. The approach, arachnoid dissection, and angles of attack are carefully planned after accounting for the aneurysm dome projection, precise location of the aneurysm neck and perforators, and the presence or absence of subarachnoid hemorrhage.
Keywords: Anterior cerebral artery, Cerebrovascular neurosurgery, Intracranial aneurysm, Microneurosurgery, Neuroanatomy
INTRODUCTION
Aneurysms of the proximal, precommunicating, or A1 segment of the anterior cerebral artery (ACA) are rare with a reported incidence of 0.59–4% and, therefore, are rarely reported in the literature.[
MATERIALS AND METHODS
Anatomic dissections were performed at Dr. Albert Rhoton’s lab (Gainsville, Fl, USA) on a formalin-fixed adult cadaveric head, in which the vessels were injected with silicone dyes (arteries in red and veins in blue). A Midas Rex high-speed drill, Carl Zeiss microscope, and microsurgical instruments were used to complete a right pterional craniotomy and Sylvian fissure split. Photographs were taken using a Nikon D7200 camera with a Micro-NIKKOR 40 mm F2.8 lens and a ring light flash. The camera was set to a diaphragm velocity of 20, a shutter speed of 1/200 and ISO 205, and the ring light flash set to 1/128.
The microsurgical management of A1 aneurysms is illustrated with two cases that underwent successful microsurgical clipping. Intraoperative photographs were obtained on a TIVATO surgical microscope (Carl Zeiss, Oberkochen, Germany) using a 125 mm video adaptor and a Blackmagic Micro Cinema Camera: 1920 × 1080 p24fps (Blackmagic Design, Port Melbourne, Victoria, Australia).
RESULTS
Microsurgical anatomy
The internal carotid artery (ICA) bifurcates right under the anterior perforated substance into its terminal branches: the middle cerebral artery (MCA), which courses laterally, and the ACA, which is of smaller diameter and courses medially and slightly anteriorly over the optic nerve and chiasm and under the medial olfactory stria before entering the interhemispheric fissure and joining the contralateral ACA through the anterior communicating artery (AComA) [
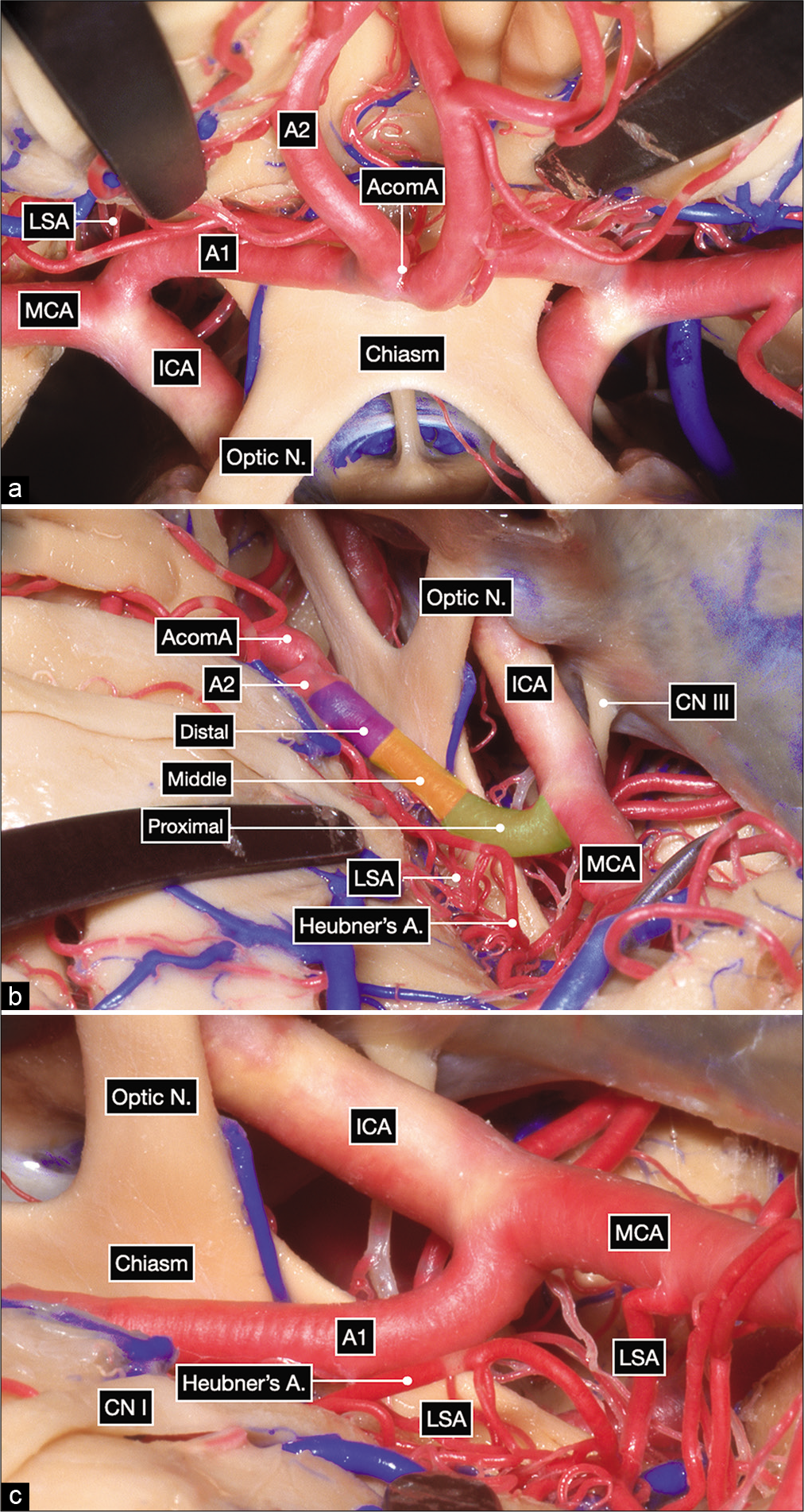
Figure 1:
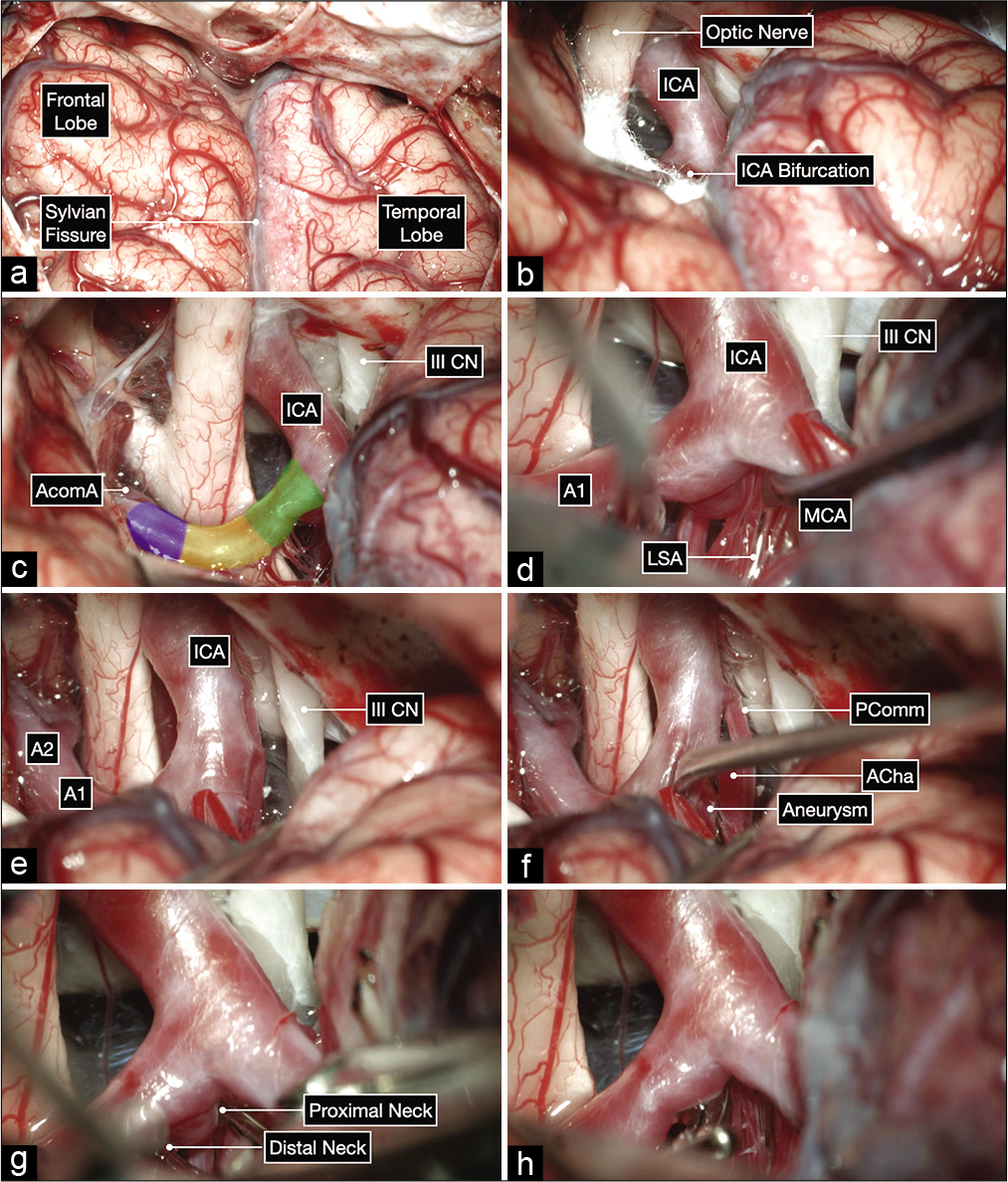
Anatomic dissection in a formalin-fixed and silicone-injected cadaveric head. (a) Anterior view after elevating the frontal lobes showing the bilateral optic nerves, optic chiasm and bilateral carotid bifurcations. The proximal, middle and distal subsegments of the first (precommunicating or A1) segment of the anterior cerebral artery (ACA) are delineated on the right side. (b and c) Surgeon’s perspective in a right pterional craniotomy after exposing the internal carotid artery bifurcation. The A1 subsegments are colored as follows: proximal in green, middle in yellow and distal in purple.
Our anatomic dissections revealed bilateral A1 segments of similar external caliber. However, hypoplasia of one segment with contralateral dominance is not uncommon, especially in patients with A1 or AComA aneurysms. The recurrent artery of Heubner, which typically branches off the ACA within 5 mm from the AComA, was found to originate from the proximal A2 (postcommunicating) segment of the ACA bilaterally in our anatomic specimen [
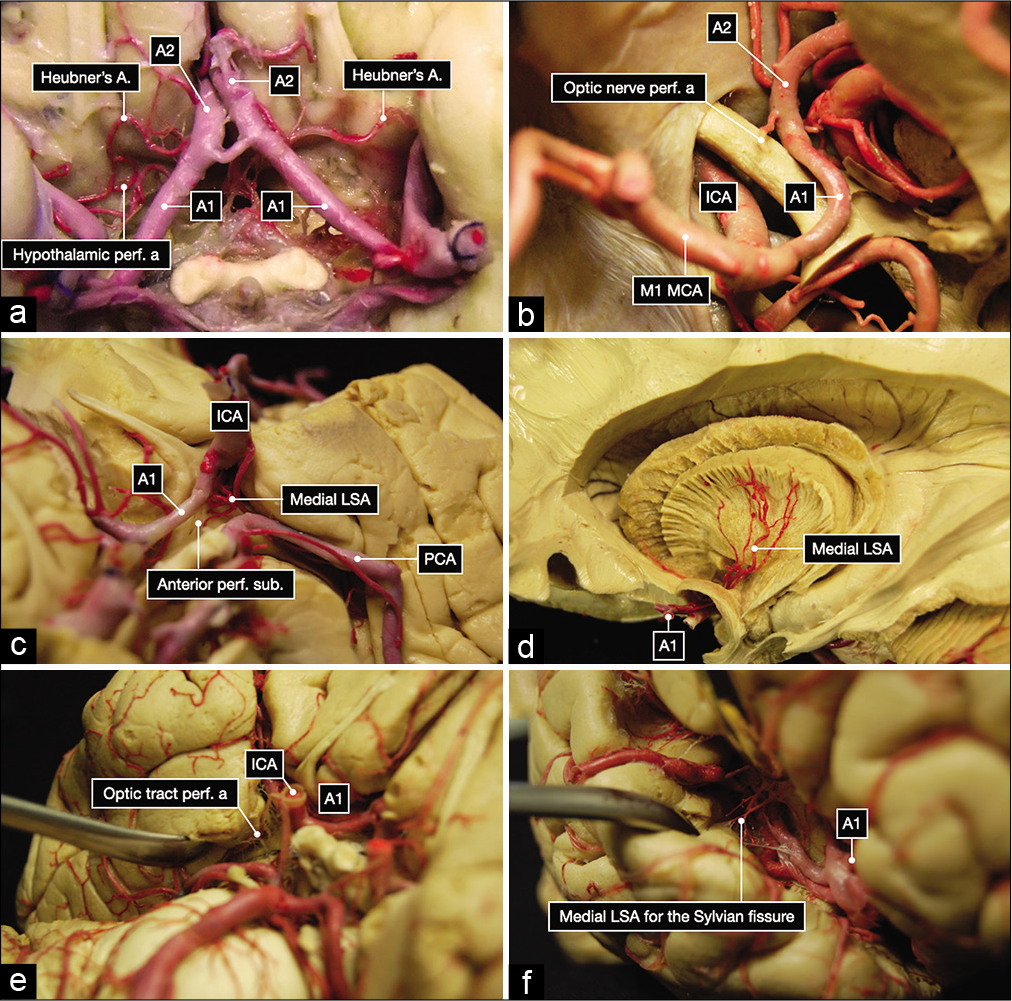
Figure 2:
Anatomic dissection of the subarachnoid cisterns performed in a formalin-fixed and silicone-injected brain. (a) Overview of the A1 and A2 segment of the anterior cerebral arteries, Heubner’s arteries, and perforating arteries to the hypothalamus from the right A1 segment. (b) Perforating artery to the left optic nerve. (c) Medial lenticulostriate arteries (LSA) to the left anterior perforated substance. (d) Dissection performed with Klinger’s technique showing the distribution to the internal capsule of the medial lenticulostriate arteries (LSA). (e) Perforating arteries to the left optic tract. (f) Perforating arteries to the right Sylvian fissure.
From a microneurosurgical standpoint, the A1 segment of the ACA can be further subdivided into three subsegments [
Illustrative case 1
The first case is a 30-year-old male who presented with a Hunt and Hess grade II, Fisher grade 2 subarachnoid hemorrhage (SAH) [
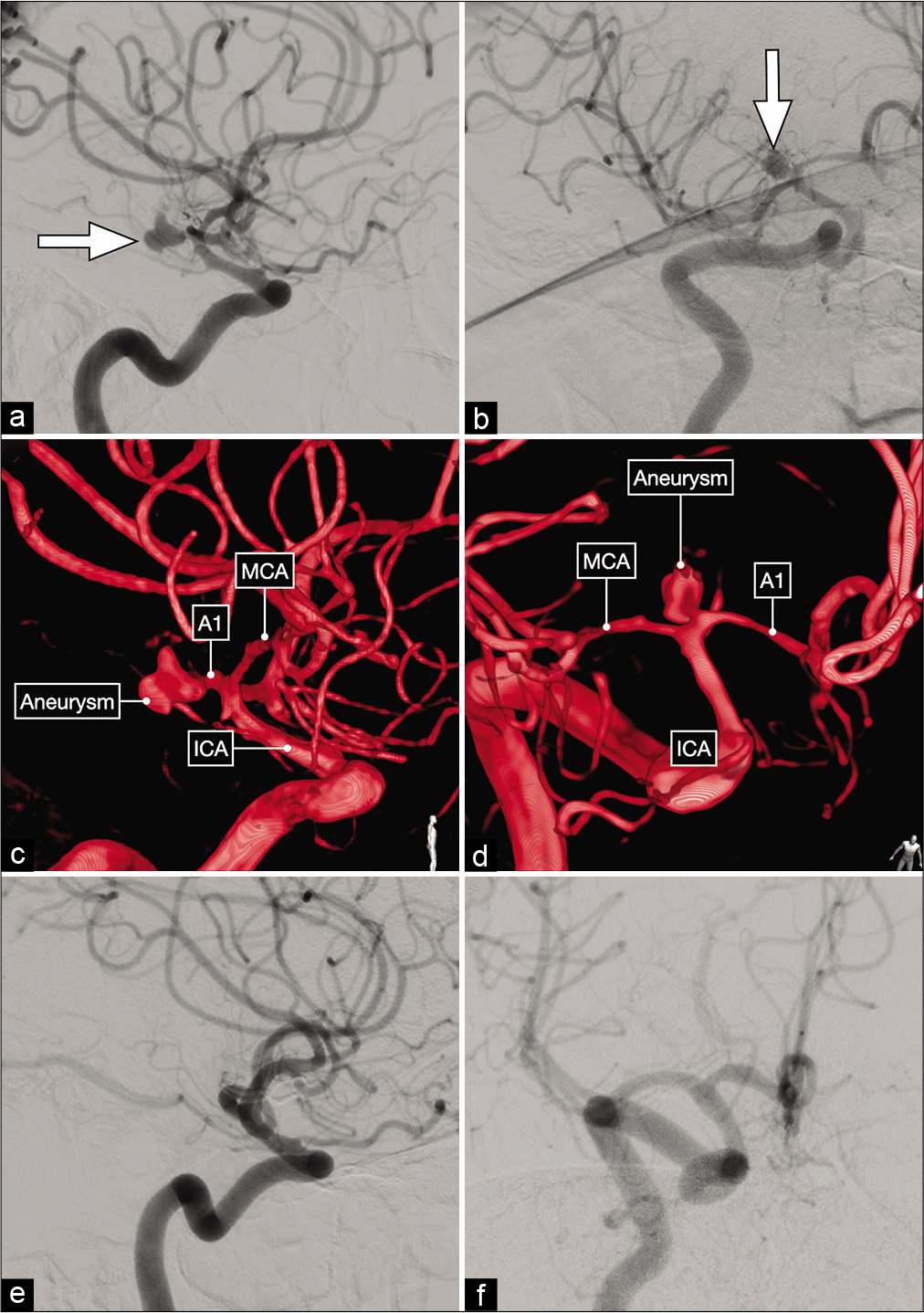
Figure 3:
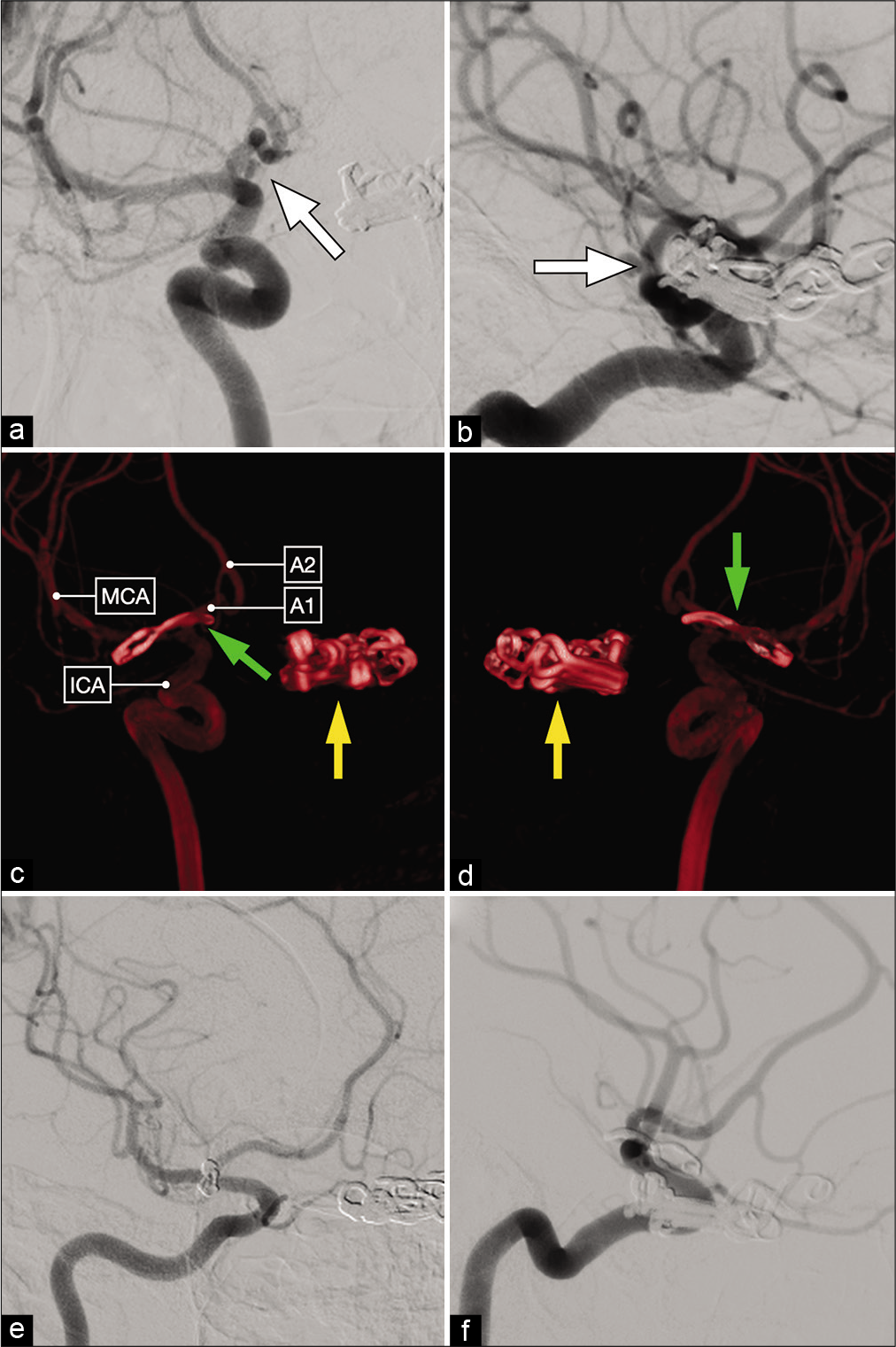
Lateral (a) and anterior oblique (b) views of a diagnostic cerebral angiogram showing a right-sided, dysmorphic, posteriorly-projecting A1 segment aneurysm (white arrow). (c and d) Three-dimensional reconstructions that better delineate the exact origin, neck and projection of this aneurysm on the proximal A1 subsegment. (e and f) Postoperative cerebral angiograms showing complete occlusion of the aneurysm and good flow across the ACA.
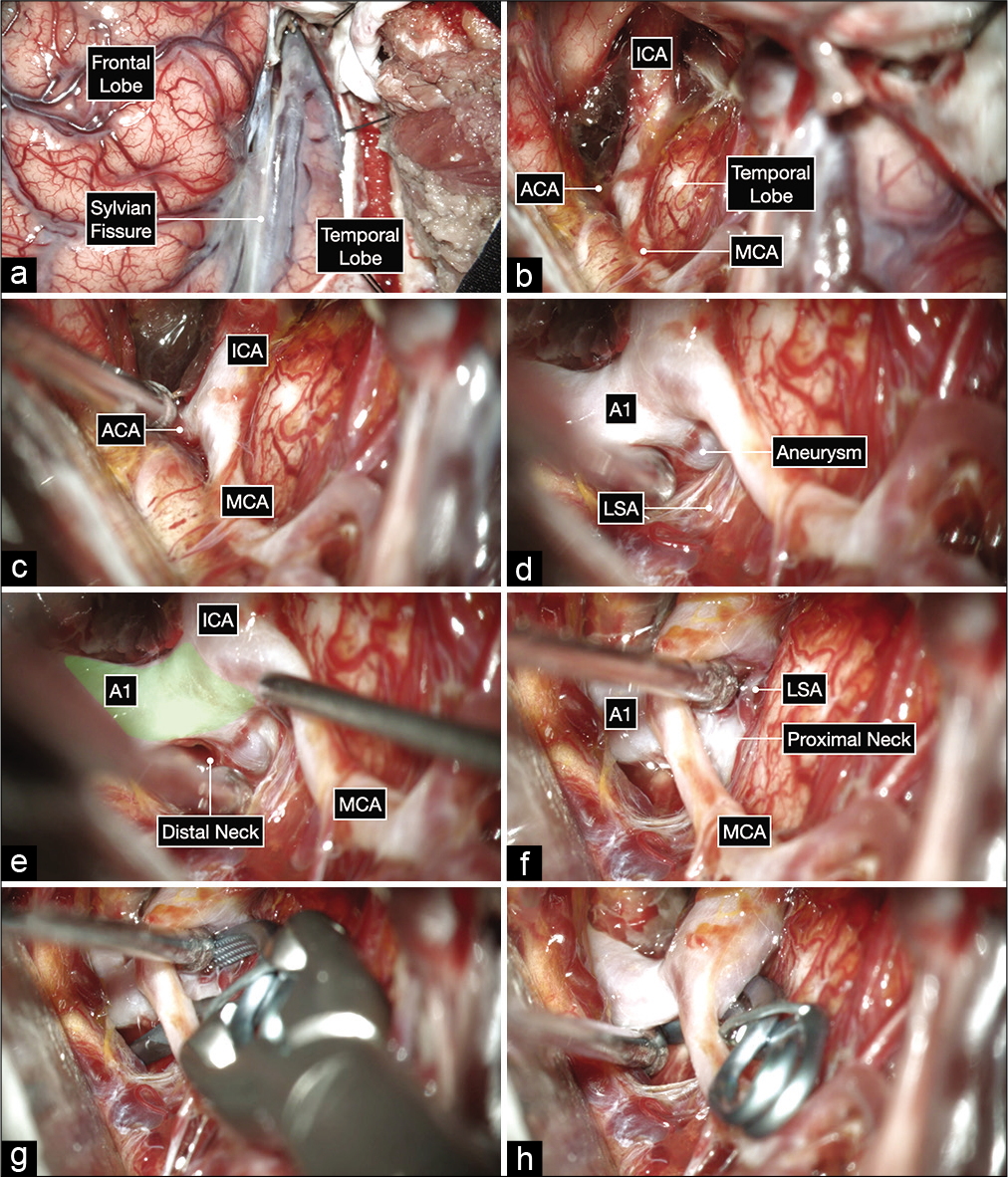
Figure 4:
Intraoperative microphotographs of the first case. (a) A right pterional craniotomy was performed to expose the right Sylvian fissure and adjacent frontal and temporal lobes. (b and c) A wide Sylvian fissure and arachnoid dissection exposed the optic nerve and ICA bifurcation. (d-f) Further, microdissection after opening into the lamina terminalis cistern and working on the anterior and inferior surface of the A1 before exposing the aneurysm on the posterior surface of the proximal A1 subsegment (colored in green). The distal neck of the aneurysm is exposed first by working superior to the MCA and following the A1 proximally toward the ICA bifurcation. The proximal neck is then exposed by gently elevating the MCA and working inferior to it (f). This maneuver can help at identifying any hidden lenticulostriate arteries (LSA). (g and h) A 10 mm curved clip was applied parallel to A1 to occlude the aneurysm while protecting the LSAs using a trajectory under the MCA with good visualization of the proximal and distal neck of the aneurysm.
Illustrative case 2
The second case is a 38-year-old-female who presented with refractory headache and a magnetic resonance imaging was suggestive of a vascular lesion on the left carotid ophthalmic region. A DSA revealed a left-sided paraclinoid and a right-sided 6 mm A1 segment aneurysm [
Figure 5:
Anteroposterior (a) and lateral (b) views of a diagnostic cerebral angiogram showing a right-sided, posteriorly-projecting A1 segment aneurysm (white arrow). Tandem clips occluding a left-sided paraclinoid ICA aneurysm are also noted. (c and d) Postoperative 3D reconstruction showing a curved clip occluding the right-sided A1 segment aneurysm (green arrow) and multiple tandem clips occluding the left-sided paraclinoid aneurysm (yellow arrow). (e and f) Postoperative diagnostic cerebral angiogram demonstrating complete aneurysm clip occlusion.
Figure 6:
Intraoperative microphotographs of the second case. (a) A right pterional craniotomy was performed to expose the right Sylvian fissure and adjacent frontal and temporal lobes. (b and c) A wide Sylvian fissure and arachnoid dissection exposed the optic nerve, ICA bifurcation, and all subsegments of the A1: proximal (in green), middle (yellow), and distal (purple). (d-h) Meticulous arachnoid dissection skeletonized the lenticulostriate arteries (LSA) and exposed the aneurysm projecting posteriorly and medially. The proximal and distal neck of the aneurysm were also exposed and a clip was applied parallel to the parent A1 using a trajectory superior to the MCA.
DISCUSSION
Aneurysms of the A1 segment of the ACA are rare and account for <1% of all intracranial aneurysms and locate more frequently on the right side.[
Hypoplasia of the contralateral A1 segment has been reported to have an incidence of 42.8%.[
Aneurysms of the A1 segment can be missed on DSAs, because they tend to be small and project posteriorly and may have an overlapping ACA segment obscuring their view. These data were also confirmed by the series of Gill et al.[
On a series of 100 aneurysms that involved the segment ranging between the ICA bifurcation and proximal A1, Jang at al. found that proximal A1 aneurysms were smaller in size, more frequently posterior projecting, regularly involving the medial LSA, and tended to be more prone to rupture.[
Aneurysms of the A1 segment of the ACA can be classified based on their neck origin and dome projection into proximal, middle, and distal A1 aneurysms.[
A very important characteristic of A1 segment aneurysms is their close relationship with small basal perforating and lenticulostriate arteries, especially aneurysms of the proximal A1 subsegment that points posteriorly and superiorly. Basal perforating arteries arising from the proximal A1 range from 2 to 15 (8 on average) and supply the anterior perforated substance, anterior commissure, globus pallidus, anterior limb of the internal capsule, dorsal surface of the optic nerve, chiasm, and tract, suprachiasmatic region of the hypothalamus, thalamus, and Sylvian fissure, as shown in
Nearly, all A1 aneurysms can be approached with a standard pterional craniotomy. In rare cases with large, superiorly projecting aneurysms, an orbital osteotomy, and orbitozygomatic approach may be advantageous for down to up visualization and to minimize frontal lobe retraction. The Sylvian fissure is split from distal to proximal, exposing the M1 and ICA bifurcation first. Microsurgical dissection of A1 aneurysms is more complex than that of AComA and ICA bifurcation aneurysms and extra care is taken to avoid intraoperative ruptures and perforator injury. A1 aneurysms tend to have a higher risk of intraoperative rupture given that their walls are weakened and may not endure manipulation or retraction, and the aneurysm dome usually adheres to the frontal lobe. Therefore, the frontal lobe should be widely untethered with meticulous arachnoid dissection to communicate the Sylvian and carotid cisterns with the suprachiasmatic and lamina terminalis cistern using dynamic retraction. Fixed retractors can be applied if needed to hold the frontal lobe in place with gravity assistance, but never forcefully retract the frontal lobe. The A1 segment of the ACA is dissected from distal to proximal, to protect the perforators in the proximal A1 subsegment. Once the MCA, ICA bifurcation, ACA, proximal and distal aneurysm neck, and perforators have been skeletonized, the ideal clip type, angle, and trajectory are chosen.
Given their rupture risk, we recommend treatment of all A1 segment aneurysms if there is no major contraindication to surgery. The expertise of the neurovascular team remains a key step in the surgical treatment of these aneurysms, along with constant microvascular training as stressed by our group.[
In an exhaustive review of the literature, Hou et al. reported that the endovascular treatment of A1 segment aneurysms is more complex than in other locations due to the before mentioned characteristics that include small size, weakened wall, wide neck, and numerous perforators, which preclude safe, complete, and durable endovascular occlusion.[
CONCLUSION
Aneurysms of the precommunicating or A1 segment of the ACA are rare and have unique characteristics that include their weakened walls with higher rupture risk despite their usually small size, their close relationship with important perforating branches, their association with anatomic variations of the ACA, and their occurrence in the setting of multiple aneurysms. A1 aneurysms are often misdiagnosed as ICA bifurcation or AComA aneurysms or missed on diagnostic cerebral angiograms due to their small size, superior projections, and the presence of obstructive overlapping ACA loops. Oblique views and three-dimensional reconstructions are recommended. The A1 segment can be subdivided into proximal, middle, and distal subsegments. The major challenge to microsurgical clipping of A1 aneurysms are protecting the numerous surrounding perforating branches (more abundant in the proximal subsegment), which requires careful preoperative localization of the aneurysm to its specific subsegment of the A1 and a distal to proximal dissection of the A1 to skeletonize the proximal and distal neck of the aneurysm as well as the perforators. However, careful planning and meticulous microneurosurgical technique are safe, effective, and durable.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Alurkar A, Karanam LS, Nayak S, Oak S. Endovascular treatment of A1 aneurysms. A series of five cases with a brief literature review. Neuroradiol J. 2012. 25: 533-40
2. Bhaisora KS, Behari S, Prasadh G, Srivastava AK, Mehrotra A, Sahu RN. A I-segment aneurysms: Management protocol based on a new classification. Neurol India. 2014. 62: 410-6
3. Chang HW, Youn SW, Jung C, Kang HS, Sohn CH, Kwon BJ. Technical strategy in endovascular treatment of proximal anterior cerebral artery aneurysms. Acta Neurochir (Wien). 2011. 153: 279-85
4. Cho YD, Ahn JH, Jung SC, Kim CH, Kang HS, Kim JE. Coil embolization in precommunicating (A1) segment aneurysms of anterior cerebral artery. Neuroradiology. 2014. 56: 219-25
5. Choque-Velasquez J, Hernesniemi J. Microsurgical clipping of a ruptured A1 segment aneurysm. Surg Neurol Int. 2018. 9: 247
6. Czepko R, Libionka W, Lopatka P. Characteristics and surgery of aneurysms of the proximal (A1) segment of the anterior cerebral artery. J Neurosurg Sci. 2005. 49: 85-95
7. Dashti R, Hernesniemi J, Lehto H, Niemelä M, Lehecka M, Rinne J. Microneurosurgical management of proximal anterior cerebral artery aneurysms. Surg Neurol. 2007. 68: 366-77
8. de Gans K, Nieuwkamp DJ, Rinkel GJ, Algra A. Timing of aneurysm surgery in subarachnoid hemorrhage: A systematic review of the literature. Neurosurgery. 2002. 50: 336-40
9. Del Maestro M, Rampini AD, Mauramati S, Lucifero AG, Bertino G, Occhini A. Dye-perfused human placenta for vascular microneurosurgery training: Preparation protocol and validation testing. World Neurosurg. 2021. 146: e854-64
10. Ding X, Nisson PL, James WS, Lawton MT, Ren S, Jia L. Aneurysms of the proximal segment of the anterior cerebral artery: A new classification system with corresponding therapeutic options. World Neurosurg. 2017. 104: 291-302
11. Friedlander RM, Oglivy CS. Aneurysmal subarachnoid hemorrhage in a patient with bilateral A1 fenestrations associated with an azygos anterior cerebral artery. Case report and literature review. J Neurosurg. 1996. 84: 681-4
12. Gill M, Maheshwari V, Mukherjee A, Gadhavi R. Microvascular clipping of A1 segment aneurysms. Neurol India. 2019. 67: 1257-63
13. Hino A, Fujimoto M, Iwamoto Y, Oka H, Echigo T. Surgery of proximal anterior cerebral artery aneurysms. Acta Neurochir (Wien). 2002. 144: 1291-6
14. Hou K, Li G, Guo Y, Yu J. Endovascular treatment for aneurysms at the A1 segment of the anterior cerebral artery: Current difficulties and solutions. Acta Neurol Belg. 2021. 121: 55-69
15. Jang CK, Jang EW, Cho KC, Suh SH, Chung J, Kim YB. Radiographic and microsurgical characteristics of proximal (A1) segment aneurysms of the anterior cerebral artery. Neurol Sci. 2018. 39: 1735-40
16. Kataoka K, Taneda M, Asai T, Kinoshita A, Ito M, Kuroda R. Structural fragility and inflammatory response of ruptured cerebral aneurysms. A comparative study between ruptured and unruptured cerebral aneurysms. Stroke. 1999. 30: 1396-401
17. Kim MK, Lim YC. Aneurysms of the proximal (A1) segment of the anterior cerebral artery: A clinical analysis of 31 cases. World Neurosurg. 2019. 127: e488-96
18. Krishnamoorthy T, Gupta AK, Bhattacharya RN, Rajesh BJ, Purkayastha S. Anomalous origin of the callosomarginal artery from the A1 segment with an associated saccular aneurysm. AJNR Am J Neuroradiol. 2006. 27: 2075-7
19. Kumar R, Behari S, Singh K, Sahu RN, Jaiswal AK. Trilobulated fusiform aneurysm from proximal fenestrated segment of dominant A1 causing subarachnoid hemorrhage. Neurol India. 2013. 61: 315-7
20. Kwon WK, Park KJ, Park DH, Kang SH. Ruptured saccular aneurysm arising from fenestrated proximal anterior cerebral artery: Case report and literature review. J Korean Neurosurg Soc. 2013. 53: 293-6
21. Lee JM, Joo SP, Kim TS, Go EJ, Choi HY, Seo BR. Surgical management of anterior cerebral artery aneurysms of the proximal (A1) segment. World Neurosurg. 2010. 74: 478-82
22. Lehecka M, Niemelä M, Hernesniemi J. Surgical management of anterior cerebral artery aneurysms of the proximal (A1) segment. World Neurosurg. 2010. 74: 439-40
23. Luzzi S, Del Maestro M, Galzio R. Microneurosurgery for paraclinoid aneurysms in the context of flow diverters. Acta Neurochir Suppl. 2021. 132: 47-53
24. Luzzi S, Gragnaniello C, Lucifero AG, Del Maestro M, Galzio R. Microneurosurgical management of giant intracranial aneurysms: Datasets of a twenty-year experience. Data Brief. 2020. 33: 106537
25. Luzzi S, Gragnaniello C, Lucifero AG, Del Maestro M, Galzio R. Surgical management of giant intracranial aneurysms: Overall results of a large series. World Neurosurg. 2020. 144: e119-37
26. Luzzi S, Lucifero AG, Baldoncini M, Del Maestro M, Elbabaa SK, Galzio R. Paraclinoid aneurysms: Outcome analysis and technical remarks of a microsurgical series. Interdiscip Neurosurg. 2022. 27: 101373
27. Maiti TK, Bir S, Konar S, Bollam P, Cuellar-Saenz HH, Nanda A. Management of proximal anterior cerebral artery aneurysms: Anatomical variations and technical nuances. World Neurosurg. 2016. 85: 85-95
28. Mäurer J, Mäurer E, Perneczky A. Surgically verified variations in the A1 segment of the anterior cerebral artery. Report of two cases. J Neurosurg. 1991. 75: 950-3
29. Minakawa T, Kawamata M, Hayano M, Kawakami K. Aneurysms associated with fenestrated anterior cerebral arteries. Report of four cases and review of the literature. Surg Neurol. 1985. 24: 284-8
30. Nandish HS, Selvapandian S, Ghosh S. Surgical significance of infra-optic course of A1 segment of anterior cerebral artery: Report of two cases. Asian J Neurosurg. 2019. 14: 927-9
31. Park HS, Choi JH, Kang M, Huh JT. Management of aneurysms of the proximal (A1) segment of the anterior cerebral artery. J Cerebrovasc Endovasc Neurosurg. 2013. 15: 13-9
32. Sato Y, Kashimura H, Takeda M, Chida K, Kubo Y, Ogasawara K. Aneurysm of the A1 segment of the anterior cerebral artery associated with the persistent primitive olfactory artery. World Neurosurg. 2015. 84: 9.e7-9
33. Sonda I, Basso L. Fenestrated A1 segment of right anterior cerebral artery associated to duplicated anterior communicating artery. Anatomy. 2015. 9: 42-4
34. Suzuki M, Onuma T, Sakurai Y, Mizoi K, Ogawa A, Yoshimoto T. Aneurysms arising from the proximal (A1) segment of the anterior cerebral artery. A study of 38 cases. J Neurosurg. 1992. 76: 455-8
35. Taylor R, Connolly ES, Duong H. Radiographic evidence and surgical confirmation of a saccular aneurysm on a hypoplastic duplicated A1 segment of the anterior cerebral artery: Case report. Neurosurgery. 2000. 46: 482-4
36. Tekkök IH, Açikgöz B. Giant aneurysm of the proximal (A1) anterior cerebral artery. Acta Neurochir (Wien). 2001. 143: 1287-92
37. Wakabayashi T, Tamaki N, Yamashita H, Saya H, Suyama T, Matsumoto S. Angiographic classification of aneurysms of the horizontal segment of the anterior cerebral artery. Surg Neurol. 1985. 24: 31-4
38. Wanibuchi M, Kurokawa Y, Ishiguro M, Fujishige M, Inaba K. Characteristics of aneurysms arising from the horizontal portion of the anterior cerebral artery. Surg Neurol. 2001. 55: 148-54
39. Yaşargil MG.editors. Microneurosurgery, Volume II: Clinical Considerations, Surgery of the Intracranial Aneurysms and Results. New York: Thieme; 1984. p.
40. Yilmaz M, Kalemci O, Yurt A, Durmaz MO, Arda NM. Treatment of aneurysms arising from the proximal (A1) segment of the anterior cerebral artery. Bosn J Basic Med Sci. 2014. 14: 8-11
41. Yu B, Wu Z, Lv X, Liu Y, Sang M. Endovascular treatment of A1 segment aneurysms of the anterior cerebral artery. Neurol India. 2010. 58: 446-8