- Department of Neurosurgery, Main Military Hospital named after N.N. Burdenko, Moscow, Russian Federation
- Department of Pathology, Military Medical Academy, Saint-Petersburg, Russian Federation
- Department of Neuropathology, N.N. Burdenko Neurosurgical Research Centre, Moscow, Russian Federation
- Department of Neurosurgery, Military Medical Academy named after S.M. Kirov, Saint-Petersburg, Russian Federation.
Correspondence Address:
Artem Stanishevskiy, Department of Neurosurgery, Main Military Hospital named after N.N. Moscow, Russian Federation.
DOI:10.25259/SNI_691_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Artem Stanishevskiy1, Andrew Jakovenko2, Marina Ryzhova3, Dmitry Svistov4, Shamil Kh Gizatullin1, Konstantin Babichev4, Evgeniy Vinogradov1, Ksenia Chemodakova4. Microstructure of embolized capsule of chronic subdural hematoma. 18-Nov-2022;13:531
How to cite this URL: Artem Stanishevskiy1, Andrew Jakovenko2, Marina Ryzhova3, Dmitry Svistov4, Shamil Kh Gizatullin1, Konstantin Babichev4, Evgeniy Vinogradov1, Ksenia Chemodakova4. Microstructure of embolized capsule of chronic subdural hematoma. 18-Nov-2022;13:531. Available from: https://surgicalneurologyint.com/surgicalint-articles/12015/
Abstract
Background: Chronic subdural hematomas (cSDHs) are frequent and potentially life-threatening neurosurgical conditions affecting, first of all, elderly. Few treatment options are available ranging from observation to removal thought large craniotomy. However, currently, there is tendency to minimize surgical aggression, especially considering poor general condition of elderly patients. Thus, one of gaining popularity method of neurointerventional treatment of cSDHs is medial meningeal artery (MMA) embolization. To date, large series of cases published describing favorable outcomes of this treatment approach. At the same time, few reports are available that describe microstructural changes in cSDH’s capsule after embolization; meanwhile, no exact effect of embolization on pathophysiology of hematoma was determined.
Case Description: Through current paper, we present two cases of cSDH that has previously undergone embolization of MMA, after which cSDHs have been operated through minicraniotomy due to complications after artery embolization. Microstructural changes of hematoma’s capsule are described and discussed.
Conclusion: Histological changes in embolized capsule suggest embolization of MMA as a valuable method for treatment of cSDHs.
Keywords: Capsule, Chronic subdural hematoma, Embolization, Microstructure
BACKGROUND
Chronic subdural hematomas (cSDHs) are widespread intracranial lesions predominantly affecting elderly and characterized by intermittent stroke-like presentation with both general symptoms and focal signs. Surgical removal of hematoma in most cases leads to complete resolution of symptoms. During past decades, there has been a paradigm shift from craniotomies with resection of inner and outer cSDH’s membranes toward minimally invasive treatment strategies[
Current paper describes two cases of cSDH which was previously treated by MMA embolization with n-butyl-cyanoacrylate (nBCA) followed by minicraniotomy with partial resection of embolized capsule of cSDH. A thorough histological examination of the obtained capsules was performed.
CASE PRESENTATION
Two patients with cSDHs were treated with MMA embolization, followed by minicraniotomy with partial resection of embolized capsule of cSDH.
Case 1
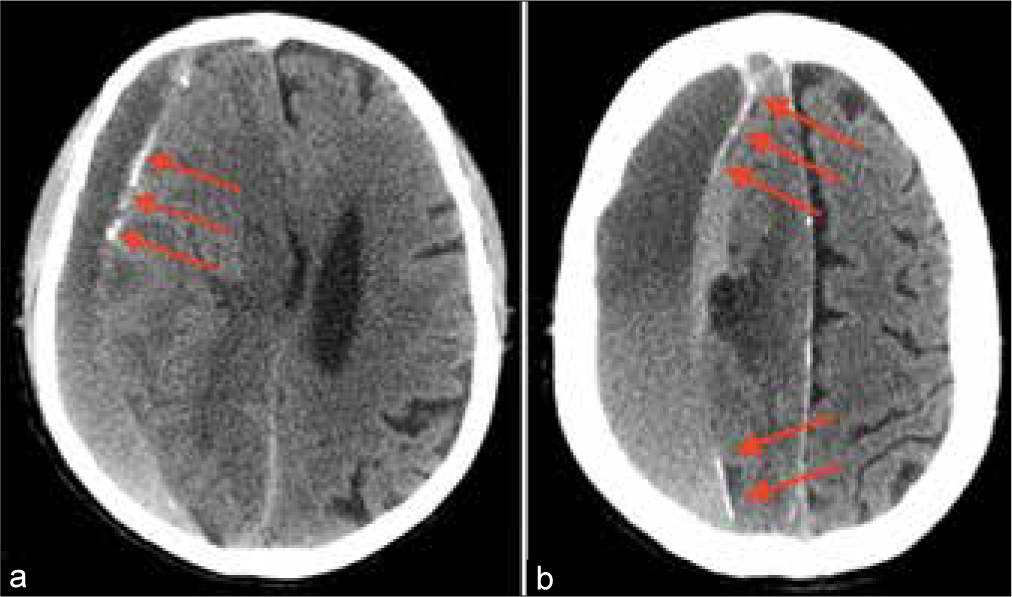
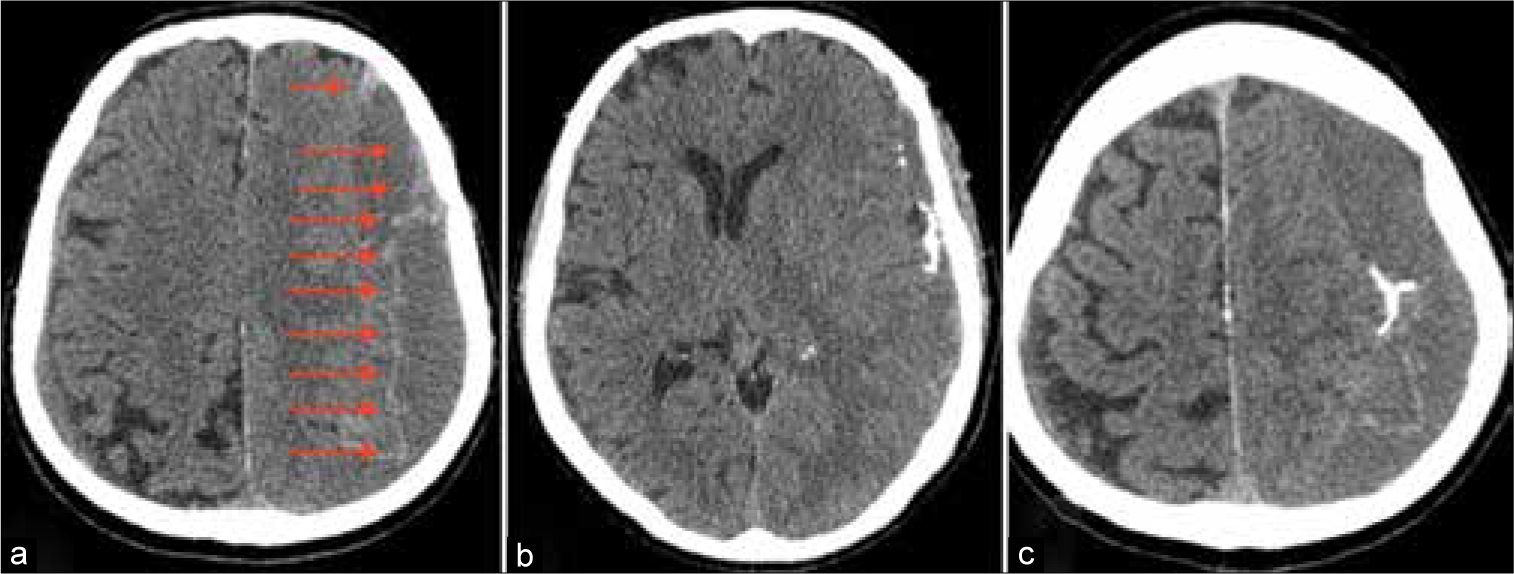
A 64-year-old male suffering from liver failure admitted to the hospital with recurrent headache and left-side hemiparesis 2 weeks after the mild head injury. Computed tomography (CT) scan revealed large right-sided cSDH. Patient’s general condition was poor as a result of prerequisite severe heart, liver, and kidney diseases. At the same time, the volume of cSDH was too high and neurological deterioration was significant for observation. Thereby, it was decided to perform MMA embolization to prevent subsequent hematoma enlargement. Postoperative CT scans revealed X-ray density enhancement of cSDH’s capsule [
After embolization, the patient was transferred to ICU to continue treatment of concomitant diseases. Unfortunately, on the 6th day, after embolization occurred a hemorrhagic stroke with large intracerebral hematoma formation in the right parietal lobe. Urgent hematoma evacuation was performed accompanied by decompressive hemicraniectomy with partial resection of cSDH capsule.
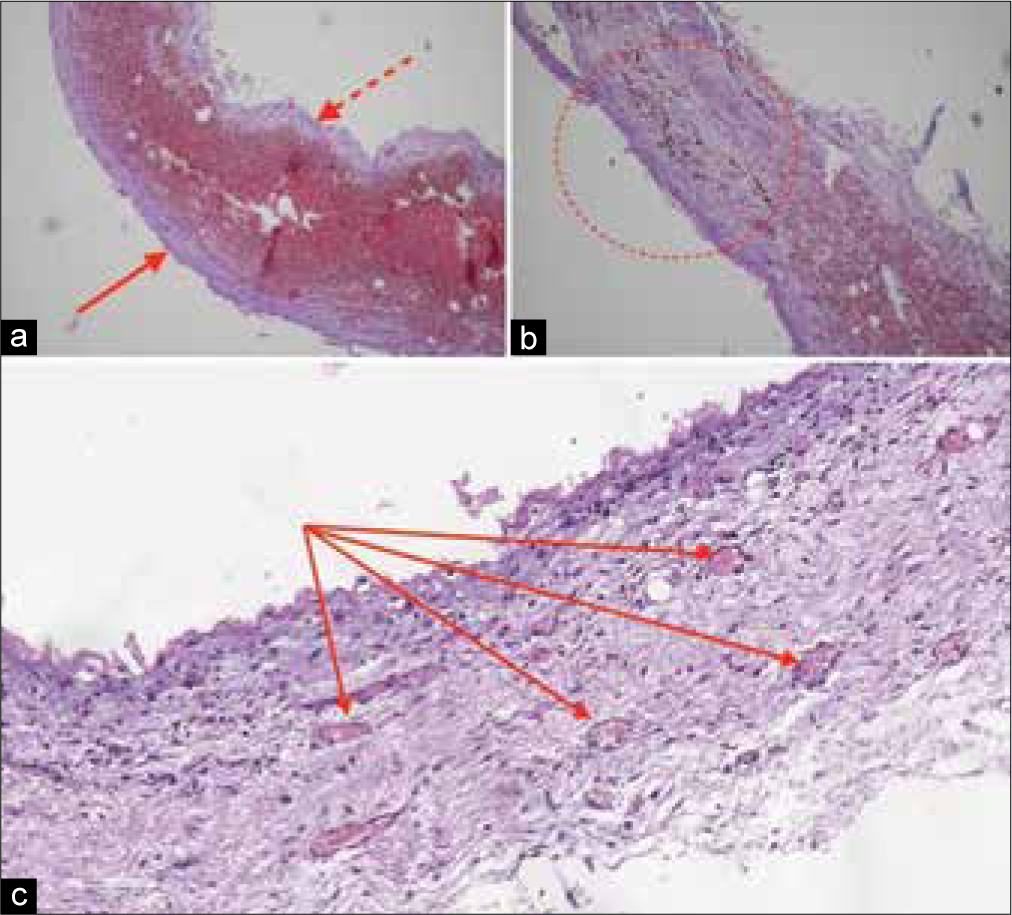
Afterward histological examination of cSDH’s capsule was performed [
Inner and outer membranes of the capsule were clearly defined. The most amount of embolized vessels were localized in outer one, which was adhere to dura mater.
Subsequent course was complicated by severe heart and kidney failure which led to refractive hypotonia and death on 34th postoperative day.
Case 2
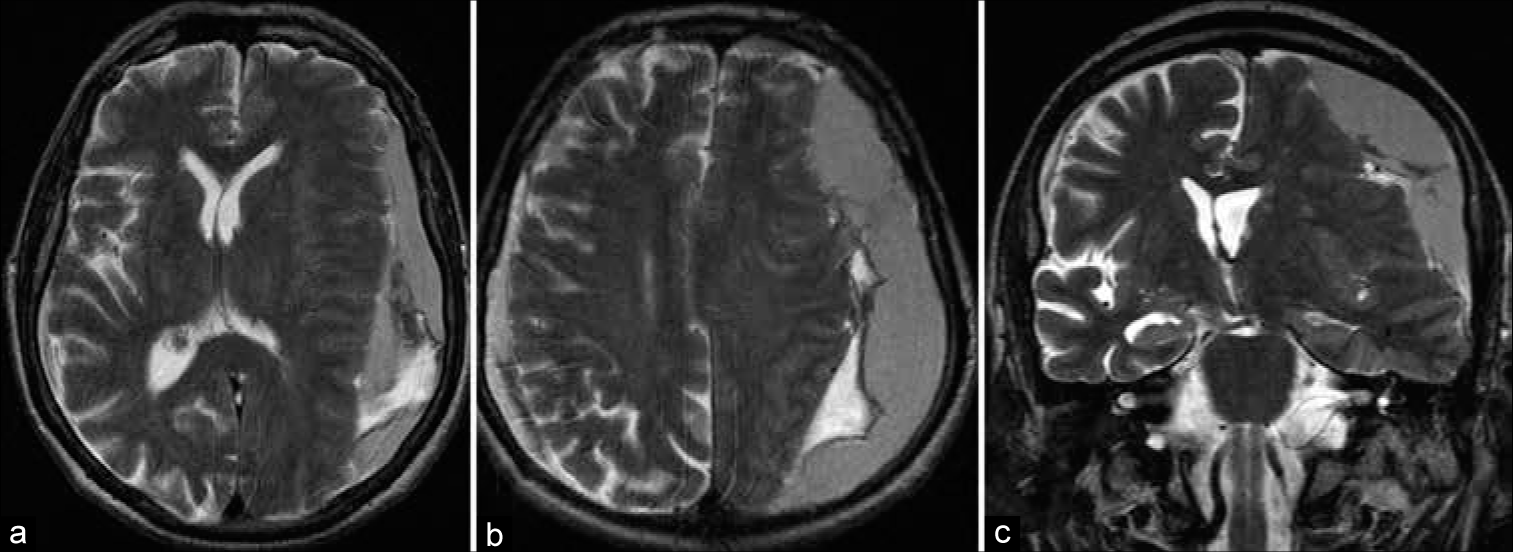
An 82-year-old male suffering from the right side weakness, speech impairment, dizziness, and gait disturbance was hospitalized at the neurosurgical department. First signs of disorder had appeared 2 weeks before admission. Neurologist on duty suspected stroke; thus, MRI scan had been performed that reveled massive left-sided cSDH [
Patient’s condition on admission was subcompensated, including Wernicke’s aphasia, ataxia, and slight right-sided hemiparesis. Prescription of tranexamic acid was contraindicated due to related atrial fibrillation. Direct surgical treatment is supposed to be associated with high risk of complications. Thus, MMA embolization was chosen as primary treatment option.
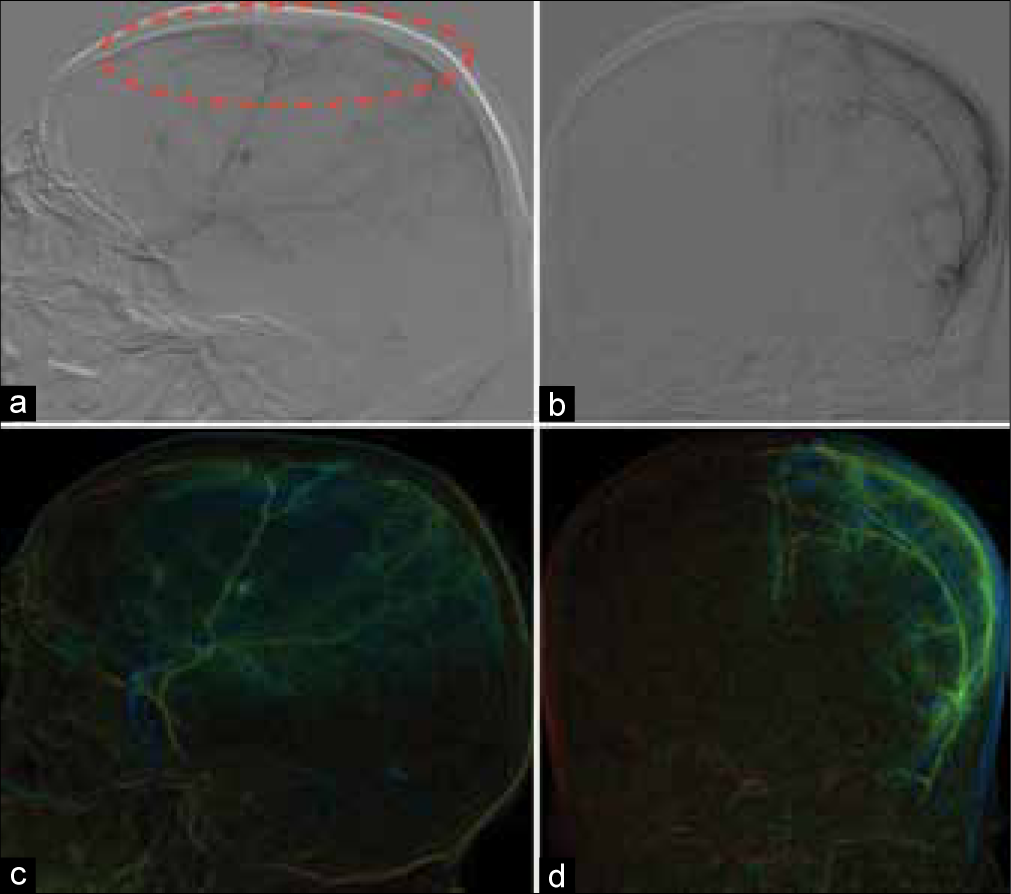
After selective catheterization of MMA, angiography was performed and clearly revealed cSDH’s capsule, which was being perfused by small caliber vessels [
Figure 4:
Selective angiography of patient 2. (a) Digital subtraction angiography, catheter in MMA, sagittal plane, capsule of cSDH highlighted by red dashed line, (b) digital subtraction angiography, coronal plane, (c and d) color reconstructions of angiograms, sagittal, and coronal plane, respectively.
Two days postoperatively, the patient was stable. CT scans revealed satisfactory pervasion of cSDH’s capsule by nBCA with several prominent clusters [
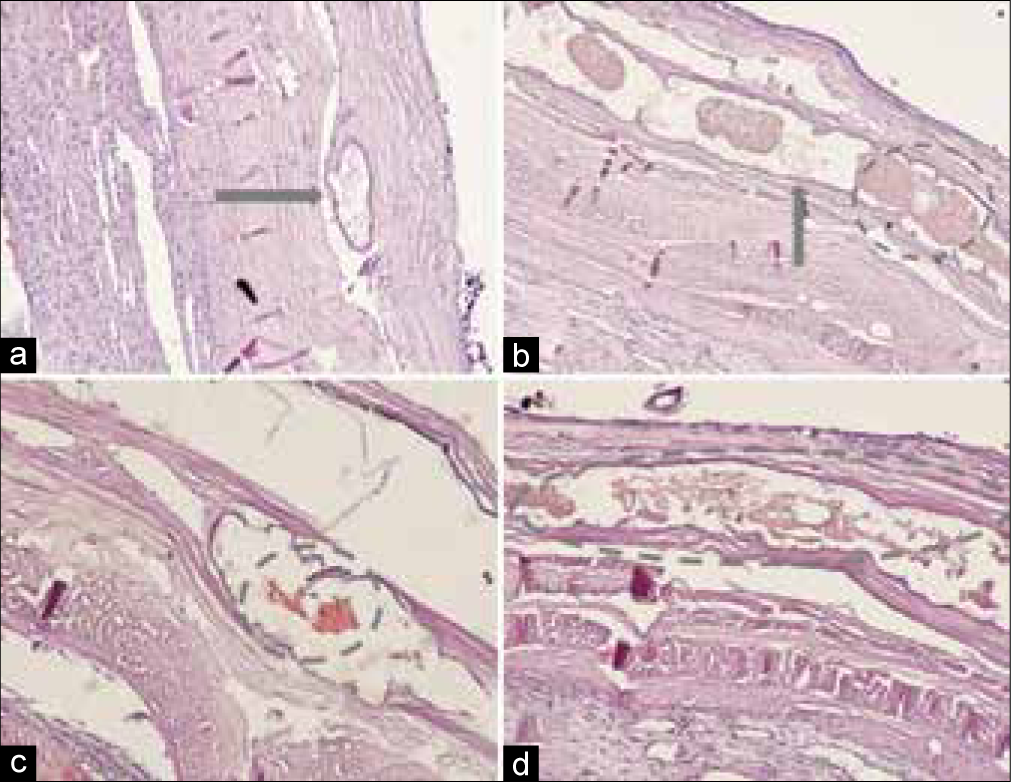
At the 3rd day of admission, neurological deterioration noted with onset of headache and impaired consciousness (GCS 12). Urgent head CT revealed no dynamics of hematoma’s volume but slight increasing of brain edema in adjacent areas. Decompensation of cSDH was proposed and decision made to evacuate hematoma through minicraniotomy. Surgery was uneventful, small piece of hematoma capsule with cluster of nBCA according to the previous CT scans was incised and histologically examined [
Figure 6:
Histological examination of cSDH’s capsule stained with hematoxylin and eosin. A – cross section of embolized dural vessel (gray arrow). (b) Longitudinal section of embolized dural vessel, nBCA fragments (gray arrow) and clumped red blood cells (dashed circle). (c and d) nBCA within dural vessels (dashed circles).
Postoperative course of patient 2 was uneventful; he was discharged at postoperative day 7 without any neurological deficit.
DISCUSSION
According to Rand, cSDHs capsule consists of two membranes: the outer (on the dural site) and the inner (on the arachnoid site).[
Despite numerous histological and ultramicroscopic studies, the exact pathomorphology and pathophysiology of CSHs remain controversial. One theory proposes that blood in the thin space between arachnoid and dura, through profound inflammatory response, leads to creation of highly vascularized outer subdural membrane attached to the dura and an inner collagenous membrane up against the brain arachnoid.[
Scanning electron microscopy revealed changes in cSDH’s fibrocellular structure developing during its evolution.[
The other theory possesses that cSDHs are not in fact “subdural,” but “intradural.”[
Lee describing the natural history of cSDHs proposes that cSDHs could be originated from subdural hygromas in patients with suitable premorbid condition – sufficient potential subdural space.[
Osmotic theory suggests dependence of cSDH’s growth or recurrence on its osmolarity instead of origin.[
Proposed mechanism of hematoma formation has direct impact on preferable treatment strategy. Shapiro et al. demonstrated that anatomical considerations supporting rationality of MMA embolization which is preferable in cSDH.[
CONCLUSION
Our findings accompanied with published data on pathophysiology of cSDHs that suggest MMA embolization as a valuable treatment method.
Declaration of patient consent
Patients’ consent not required as patients’ identities were not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest
References
1. Chen JW, Xu JC, Malkasian D, Perez-Rosendahl MA, Tran DK. The mini-craniotomy for cSDH revisited: New perspectives. Front Neurol. 2021. 12: 660885
2. Di Cristofori A, Remida P, Patassini M, Piergallini L, Buonanno R, Bruno R. Middle meningeal artery embolization for chronic subdural hematomas. A systematic review of the literature focused on indications, technical aspects and future possible perspectives. Surg Neurol Int. 2022. 13: 94
3. Lee KS. Natural history of chronic subdural haematoma. Brain Inj. 2004. 18: 351-8
4. Link TW, Boddu S, Paine SM, Kamel H, Knopman J. Middle meningeal artery embolization for chronic subdural hematoma: A Series of 60 Cases. Neurosurgery. 2019. 85: 801-7
5. Moskala M, Goscinski I, Kaluza J, Polak J, Krupa M, Adamek D. Morphological aspects of the traumatic chronic subdural hematoma capsule: SEM studies. Microsc Microanal. 2007. 13: 211-9
6. Nia AM, Srinivasan VM, Lall RR, Kan P. Middle meningeal artery embolization for chronic subdural hematoma: A national database study of 191 patients in the United States. World Neurosurg. 2021. 153: e300-7
7. Potapov AA, Likhterman LB, Kravchuk AD, editors. Chronic Subdural Hematomas. Antidor Moscow. 1997. p.
8. Rand CW. Chronic subdural hematoma: Report of seven cases. Arch Surg. 1927. 14: 1136-65
9. Saito H, Tanaka M, Hadeishi H. Angiogenesis in the septum and inner membrane of refractory chronic subdural hematomas: Consideration of findings after middle meningeal artery embolization with low-concentration n-butyl-2-cyanoacrylate. NMC Case Rep J. 2019. 6: 105-10
10. Shapiro M, Walker M, Carroll KT, Levitt MR, Raz E, Nossek E. Neuroanatomy of cranial dural vessels: Implications for subdural hematoma embolization. J Neurointerv Surg. 2021. 13: 471-7
11. Stanishevskiy AV, Babichev KN, Vinogradov EV, Gizatullin SK, Svistov DV, Kandyba DV. Embolizatsiya srednei obolochechnoi arterii kak metod lecheniya khronicheskikh subdural’nykh gematom. Seriya klinicheskikh sluchaev i obzor literatury [Middle meningeal artery embolization for chronic subdural haematoma. Case series and literature review]. Zh Vopr Neirokhir Im N N Burdenko. 2021. 85: 71-9
12. Thomas PA, Marshman LA, Rudd D, Moffat C, Mitchell PS. Growth and resorption of chronic subdural hematomas: Gardner, weir, and the osmotic hypothesis revisited. World Neurosurg. 2019. 132: e202-7
13. Yamashima T. The inner membrane of chronic subdural hematomas: Pathology and pathophysiology. Neurosurg Clin N Am. 2000. 11: 413-24