- Department of Neurosurgery, Sapporo Teishinkai Hospital, Sapporo, Hokkaido, Japan.
DOI:10.25259/SNI_300_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Nakao Ota, Yasuaki Okada, Kosumo Noda, Rokuya Tanikawa. Microsurgical embolectomy with superficial temporal artery-middle cerebral artery bypass for acute internal carotid artery dissection: A technical case report. 01-Aug-2020;11:223
How to cite this URL: Nakao Ota, Yasuaki Okada, Kosumo Noda, Rokuya Tanikawa. Microsurgical embolectomy with superficial temporal artery-middle cerebral artery bypass for acute internal carotid artery dissection: A technical case report. 01-Aug-2020;11:223. Available from: https://surgicalneurologyint.com/surgicalint-articles/10177/
Abstract
Background: Dissection of the internal carotid artery (ICA) is an important cause of stroke. Intravenous alteplase administration and mechanical thrombectomy have been strongly recommended for selected patients with acute ischemic stroke. However, the efficacy and safety of these treatments for ischemic stroke due to ICA dissection remain unclear. Here, we report a case of acute ICA dissection successfully treated by microsurgical embolectomy.
Case Description: A 40-year-old man presented with sudden left hemiparesis and in an unconscious state, with a National Institutes of Health Stroke Scale score of 14. Preoperative radiologic findings revealed an ICA dissection from the extracranial ICA to the intracranial ICA and occlusion at the superior-most aspect of the ICA. A dissection at the superior-most aspect of the ICA occlusion could not be confirmed; therefore, a surgical embolectomy with bypass was initiated. It became apparent that the superior ICA occlusion was not due to dissection but rather to an embolic occlusion; therefore, we undertook a surgical embolectomy and cervical ICA ligation with a double superficial temporal artery-middle cerebral artery bypass. The postoperative course was uneventful and, at the 6-month follow-up, the Modified Rankin Scale score for this patient was 1.
Conclusion: Surgical embolectomy with or without bypass can safely treat acute ischemic stroke due to an ICA dissection that cannot be distinguished between a dissecting occlusion and an embolic occlusion. Thus, it may be considered as an alternative option for patients in whom mechanical thrombectomy has failed or for those who are ineligible for mechanical thrombectomy.
Keywords: Acute ischemic stroke, Internal carotid artery dissection, Pathologic finding, Superficial temporal artery-middle cerebral artery bypass, Surgical embolectomy
INTRODUCTION
Dissection of the internal carotid artery (ICA) is an important cause of stroke in younger patients.[
METHODS
Presentation
This study was approved Institutional Review Board with patients consent. A 40-year-old man with a history of uncontrolled hypertension presented with sudden left-sided hemiparesis. He was unconscious, with a National Institutes of Health Stroke Scale score of 14. Magnetic resonance imaging (MRI) was immediately performed [
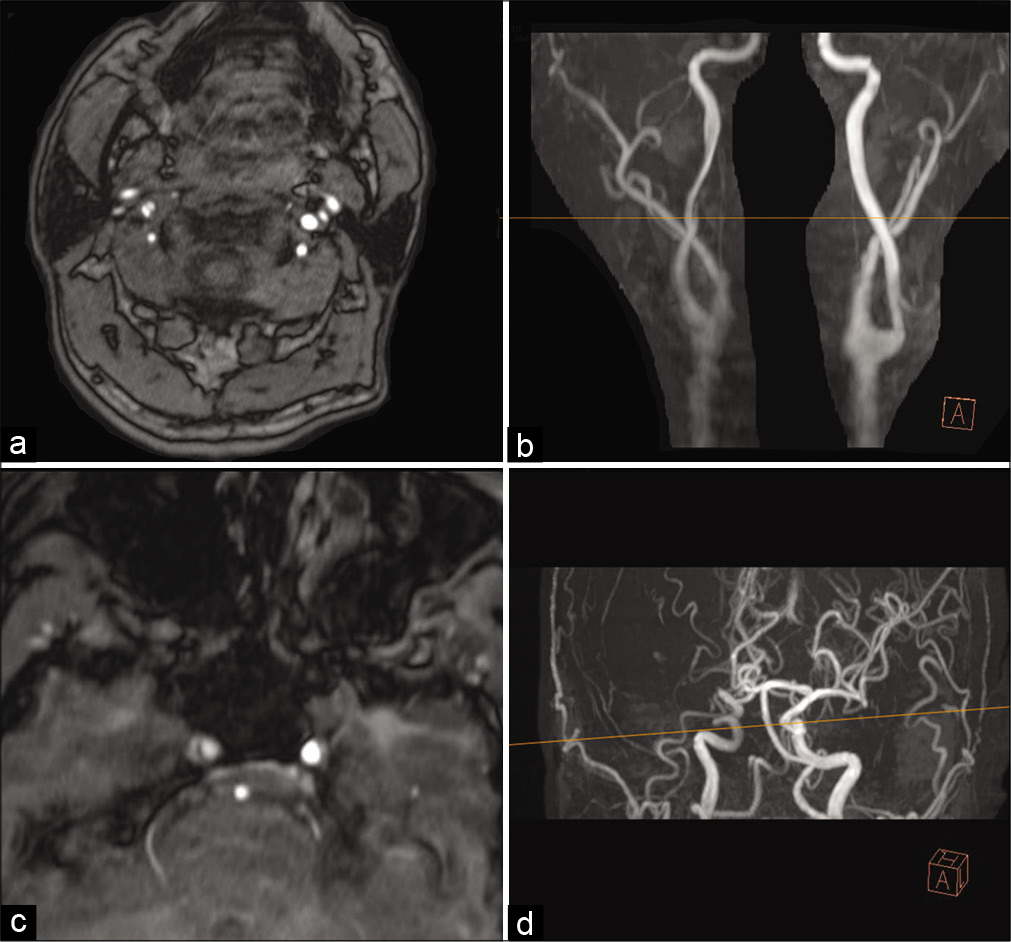
Figure 1:
Time-of-flight (TOF) magnetic resonance angiography at onset. (a) A double lumen sign is identified in the C7 segment of the internal carotid artery (ICA). (b) 3D TOF showed narrowing of the C7 portion of the ICA. The line represents the reference line of figure (a). (c) A double lumen sign is identified in the C3 segment of the ICA. (d) The line represents the reference line of figure (c).
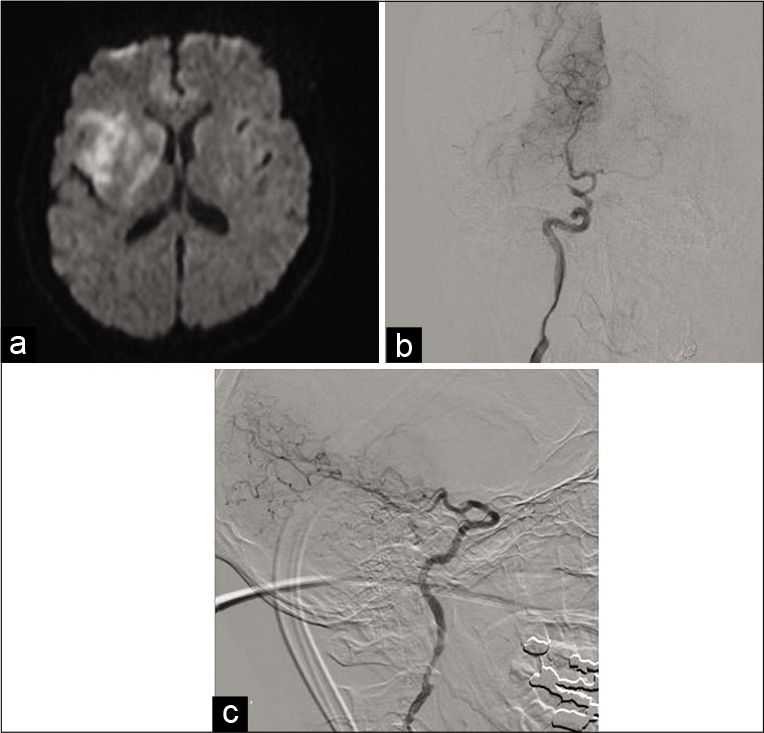
Figure 2:
Diffusion-weighted image (DWI) and digital subtraction angiography. (a) DWI showed infarction of the posterior limb of the internal capsule, basal ganglia, and the insular and frontal cortices. (b and c) Digital subtraction angiography showed C7 narrowing and the superior-most aspect of the ICA occlusion.
Treatment strategy
We deemed that occlusion of the superior-most aspect of the ICA was either due to an embolic occlusion or an arterial dissection that developed in the C1 segment of the ICA. We decided that a dissecting occlusion could not be excluded in this case for the following reasons: (1) on MRI, the dissection appeared to spread along the C3 segment of the ICA and (2) the anterior choroidal artery was included in the infarction. Hence, we could not exclude the possibility of the dissection spreading to the C1 segment of the ICA. Based on these findings, a surgical embolectomy with bypass was planned.
Surgical approach (video)
A pterional approach which involved harvesting the frontal and parietal branch of the superficial temporal artery (STA) was performed. Following a frontotemporal craniotomy, the Sylvian fissure was opened widely to identify the M1–M3 segments of the middle cerebral artery (MCA). The MI segment appeared black in color and was occluded. Initially, the STA of the M3 segment of the MCA bypass secured distal blood flow. Then, an additional dissection was performed to identify the ICA. The posterior communicating artery was secured; however, the anterior choroidal artery was occluded by a white thrombus. Furthermore, the C1 segment of the ICA showed no evidence of dissection. The anterior choroidal artery was recanalized by massage. An arteriotomy was then performed on the M2 segment of the MCAs superior trunk to determine whether the occlusion was either due to an embolus or the development of an ICA dissection. In our patient, the occlusion was embolism related; therefore, the M1-M2 segment was recanalized by removing the embolus with no evidence of an MCA dissection. A parietal-branch STA-MCA bypass was undertaken, after which the MCA pressure was monitored through the other small branch of the STA. MCA pressure monitoring was performed to determine whether the cerebral blood flow was adequate, or whether the ICA was occluded.[
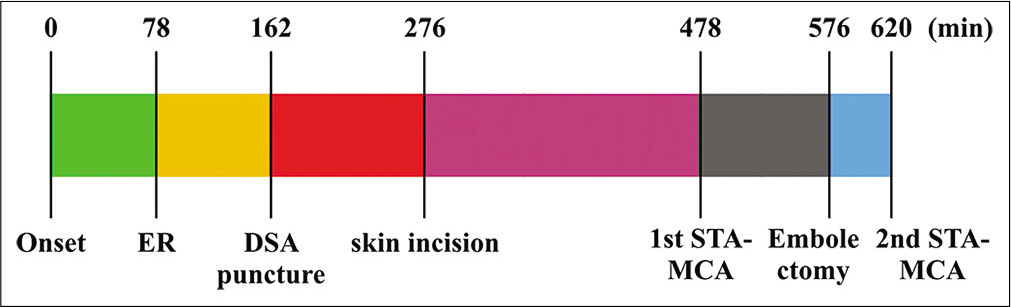
Figure 3:
Treatment time course. Surgical embolectomy began 198 min after the patient was admitted to the emergency room. Superficial temporal artery-middle cerebral artery bypass was completed 2 h after skin incision (6.7 h after onset), and complete recanalization was established 8.3 h (498 min) after onset.
Postoperative course
Postoperative diffusion-weighted imaging showed no additional infarction [
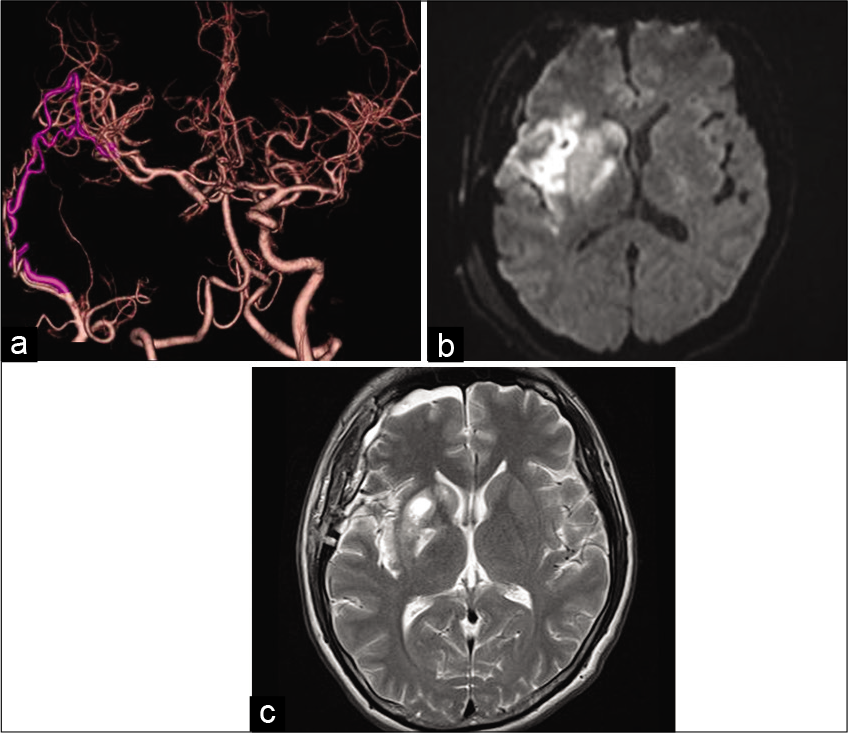
Figure 4:
Postoperative imaging. (a) computed tomography angiography showed good patency of the superficial temporal artery-middle cerebral artery bypass and recanalization at the superior-most aspect of the internal carotid artery. (b) Diffusion- weighted image showed no additional infarction. (c) T2 imaging 2 weeks postonset showed hyperintensity on the posterior limb of the internal capsule and basal ganglia.
Pathological findings
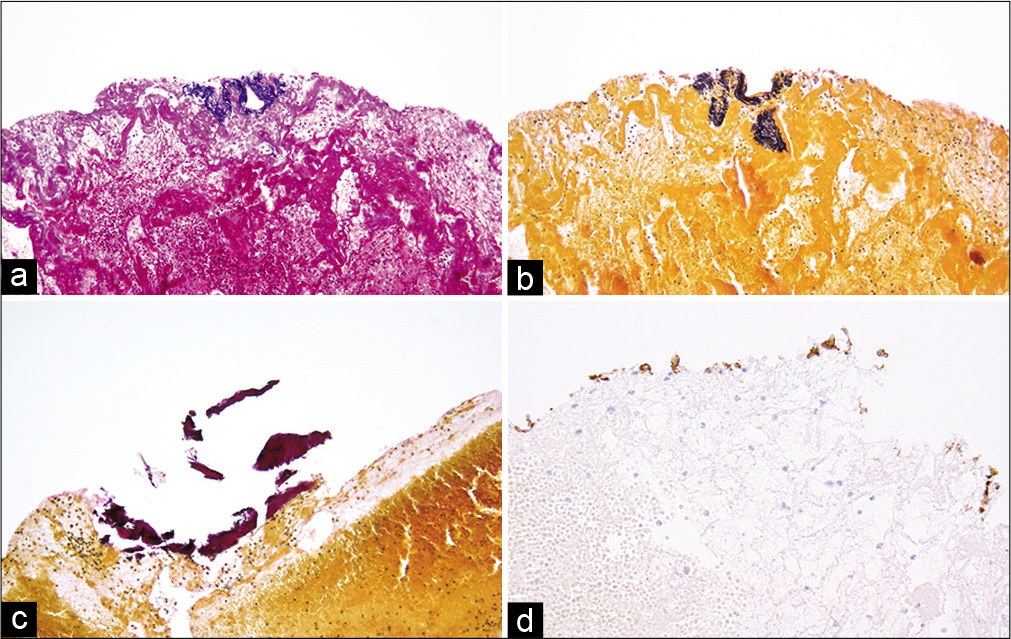
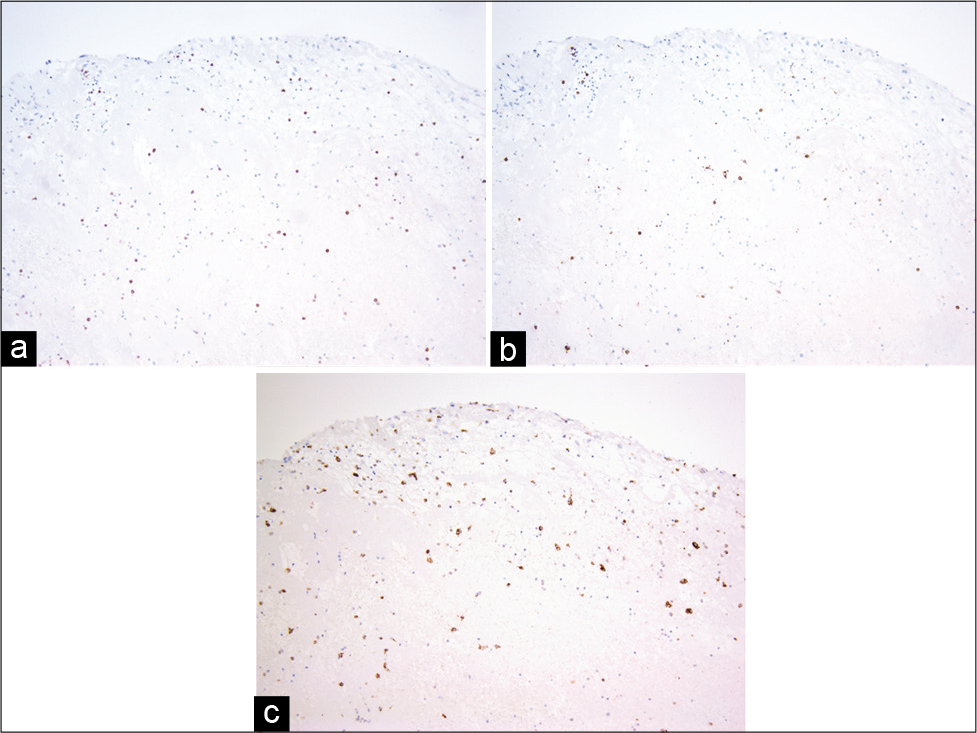
Pathological findings of the removed thrombus using Elastica-HE, Elastica van Gieson, CD3, CD20, CD34, and CD 68 stains showed a red thrombus that included elastic fibers, calcification, and infiltration of T and B cells and macrophages [
DISCUSSION
While it was not apparent in our patient until after beginning the intervention, the dissection had not developed not only in the intradural segment of the ICA but also in the MCA. Hence, treatment could have been undertaken through mechanical thrombectomy with stenting of the dissection site. However, our case is important in this regard for the following reasons. First, an initial diagnosis of the dissection length of the ICA in the acute phase and a treatment strategy was not established. Second, surgical embolectomy can be used as a third-line treatment strategy for acute embolic stroke.
Assessing ICA dissection length
Among reported cases concerning ICA dissection, many cases have involved cervical ICA dissection. Ischemic stroke due to cervical ICA dissection in young and middle-aged patients comprises 8–25% of ischemic stroke incidence,[
Treatment of embolic stroke due to ICA dissection
One study reported eight cases of mechanical thrombectomy for ischemic stroke due to ICA dissection from the Merci Registry, which is a prospective, multicenter postmarket database comprising patients treated with the Merci Retriever thrombectomy device.[
Surgical embolectomy as a third-line treatment strategy involving recanalization following embolic stroke
There is strong evidence for undertaking endovascular revascularization for acute embolic stroke following administration of intravenous tPA. However, in some cases, this procedure cannot be performed, such as in patients with iodine enhancement allergy, access difficulties because of severe atherosclerotic change or anatomical variation, embolic stroke due to carotid plaque rupture,[
Pathophysiology of the ICA dissection
In our case, the histopathological findings concerning the embolus showed that intima, internal elastic lamina, and calcification were included in the thrombus. In addition, some inflammation cells such as T and B cell and macrophages had infiltrated. Dargazanli et al. reported that the CD3+ T-cell count in intracranial thrombi was significantly higher in atherothrombotic origin strokes compared to that in all other causes and that thrombi with high content of CD3+ cells are more likely to originate from an atherosclerotic plaque.[
Study limitations
Because this study involved a case of emergent ischemia, a detailed MRI study was insufficient in this case to determine the full extent of the diagnosis. In addition, in the future, studies should be performed involving case series or blinded protocols utilizing larger groups.
CONCLUSION
Acute ischemic stroke due to ICA dissection that cannot be distinguished from a dissecting or embolic occlusion can safely be treated using surgical embolectomy with or without bypass. This is a possible option for patients who have undergone mechanical thrombectomy or those who are ineligible for mechanical thrombectomy.
Declaration of patient consent
Institutional Review Board permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Chaves C, Estol C, Esnaola MM, Gorson K, O’Donoghue M, De Witt LD. Spontaneous intracranial internal carotid artery dissection: Report of 10 patients. Arch Neurol. 2002. 59: 977-81
2. Dargazanli C, Rigau V, Eker O, Bareiro CR, Machi P, Gascou G. High CD3+ cells in intracranial thrombi represent a biomarker of atherothrombotic stroke. PLoS One. 2016. 11: e0154945
3. Fabris F, Zanocchi M, Bo M, Fonte G, Poli L, Bergoglio I. Carotid plaque, aging, and risk factors. A study of 457 subjects. Stroke. 1994. 25: 1133-40
4. Fields JD, Lutsep HL, Rymer MR, Budzik RF, Devlin TG, Baxter BW. Endovascular mechanical thrombectomy for the treatment of acute ischemic stroke due to arterial dissection. Interv Neuroradiol. 2012. 18: 74-9
5. Goehre F, Yanagisawa T, Kamiyama H, Noda K, Ota N, Tsuboi T. Direct microsurgical embolectomy for an acute distal basilar artery occlusion. World Neurosurg. 2016. 86: 497-502
6. Inoue T, Tamura A, Tsutsumi K, Saito I, Saito N. Surgical embolectomy for large vessel occlusion of anterior circulation. Br J Neurosurg. 2013. 27: 783-90
7. Kiyofuji S, Inoue T, Hasegawa H, Tamura A, Saito I. Emergent surgical embolectomy for middle cerebral artery occlusion due to carotid plaque rupture followed by elective carotid endarterectomy. J Neurosurg. 2014. 121: 631-6
8. Matsukawa H, Tanikawa R, Kamiyama H, Tsuboi T, Noda K, Ota N. Risk factors for low-flow related ischemic complications and neurologic worsening in patients with complex internal carotid artery aneurysm treated by extracranial to intracranial high-flow bypass. World Neurosurg. 2016. 85: 49-55
9. Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018. 378: 11-21
10. Ota N, Tanikawa R, Kamiyama H, Miyazaki T, Noda K, Katsuno M. Discrepancy between preoperative imaging and postoperative pathological finding of ruptured intracranial dissecting aneurysm, and its surgical treatment: Case report. Neurol Med Chir (Tokyo). 2014. 54: 219-26
11. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K. 2018 Guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the American heart association/ American stroke association. Stroke. 2018. 49: e46-110
12. Schievink WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. 2001. 344: 898-906
13. Schwartz NE, Vertinsky AT, Hirsch KG, Albers GW. Clinical and radiographic natural history of cervical artery dissections. J Stroke Cerebrovasc Dis. 2009. 18: 416-23
14. Takemoto K, Takano K, Abe H, Okawa M, Iwaasa M, Higashi T. The new MRI modalities BPAS and VISTA for the diagnosis of VA dissection. Acta Neurochir Suppl. 2011. 112: 59-65
15. Zhang FL, Guo ZN, Liu Y, Luo Y, Yang Y. Dissection extending from extra-to intracranial arteries: A case report of progressive ischemic stroke. Medicine (Baltimore). 2017. 96: e6980