- Department of Neurosurgery, National Institute of Neurology and Neurosurgery, Mexico City, Mexico.
Correspondence Address:
R. Revuelta-Gutiérrez, Department of Neurosurgery, National Institute of Neurology and Neurosurgery, Mexico City, Mexico.
DOI:10.25259/SNI_578_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Alejandro Serrano Rubio, Héctor A. Rodríguez-Rubio, Rodrigo López-Rodríguez, Alfredo Bonilla-Suastegui, Fernando Piñón-Jiménez, Oscar Rubén Contreras-Vázquez, R. Revuelta-Gutiérrez. Microvascular decompression for hemifacial spasm: Complications after 292 procedures without neurophysiological monitoring. 22-Sep-2023;14:343
How to cite this URL: Alejandro Serrano Rubio, Héctor A. Rodríguez-Rubio, Rodrigo López-Rodríguez, Alfredo Bonilla-Suastegui, Fernando Piñón-Jiménez, Oscar Rubén Contreras-Vázquez, R. Revuelta-Gutiérrez. Microvascular decompression for hemifacial spasm: Complications after 292 procedures without neurophysiological monitoring. 22-Sep-2023;14:343. Available from: https://surgicalneurologyint.com/surgicalint-articles/12566/
Abstract
Background: Hemifacial spasm (HFS) is characterized by involuntary, progressive, and intermittent spasms in the upper and lower facial muscles. Due to the high success rate, microvascular decompression (MVD) is the treatment of choice, and intraoperative neuromonitoring (INM) is considered useful for achieving safe surgery. Still, most centers do not have this technology.
Methods: We analyzed 294 patients with HFS treated with MVD without INM. We only included patients with a neurovascular etiology while excluding other causes, such as tumors. As part of the postoperative evaluation, we assessed preoperative magnetic resonance imaging and pure-tone audiometry.
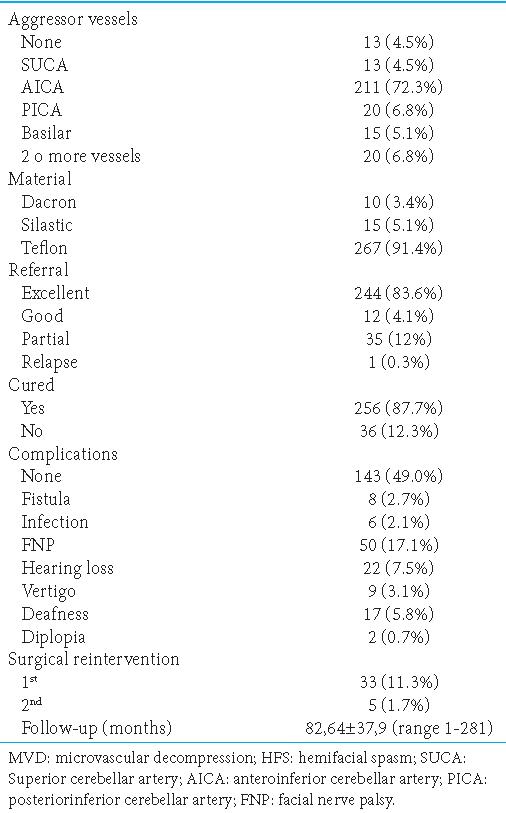
Results: The main complication was peripheral facial paralysis in 50 patients, followed by hypoacusis in 22 patients and deafness in 17 patients, associated with a failed surgical outcome (P = 0.0002). The anterior inferior cerebellar artery (AICA) was an offending vessel, and the involvement of more than one vessel was significantly associated with the development of facial nerve palsy (P = 0.01). AICA was also associated with hearing impairment (P = 0.04). Over 90% of immediate complications improve in the follow-up (6 months), and one patient did not show a cure for initial HFS.
Conclusion: MVD is the method with the highest long-term cure rates for treating HFS; however, we must inquire into the multiple factors of the patient and the surgeon to predict surgical outcomes. INM is not a must during MVD for HFS. We recommend its use depending on the availability and mainly on the surgeon’s skills, for surgeons.
Keywords: Hemifacial spasm, Intraoperative neurophysiological monitoring, Microvascular decompression, Postoperative complications
INTRODUCTION
Hemifacial spasm (HFS) is a rare condition characterized by involuntary, progressive, and intermittent spasms in the upper and lower facial muscles. It is most often unilateral (bilateral in <1% of cases) and is attributed to an aberrant vascular loop compressing the facial nerve at its emergence into the brainstem.[
MATERIALS AND METHODS
We reviewed and analyzed the clinical data and medical files of patients treated with MVD for HFS from May 1992 to December 2018; we made this study in adherence to STROBE guidelines for retrospective observational cohorts. We only included patients with a neurovascular etiology while excluding other causes, such as tumors. As part of the postoperative evaluation, we assessed all patients’ preoperative magnetic resonance imaging and pure-tone audiometry (PTA) studies. Our institutional ethics board approved this study.
Population and data collection
Outcome collection included age, sex, history of the previous medications, duration of HFS, previous medical treatments, complications, and the presence or absence of HFS at hospital discharge. HFS outcome was set on the relief of symptoms and classified according to the previously described Revuelta’s HFS score criteria.[
Surgical procedure
We performed the surgical procedure using a published method as Soriano et al. described.[
RESULTS
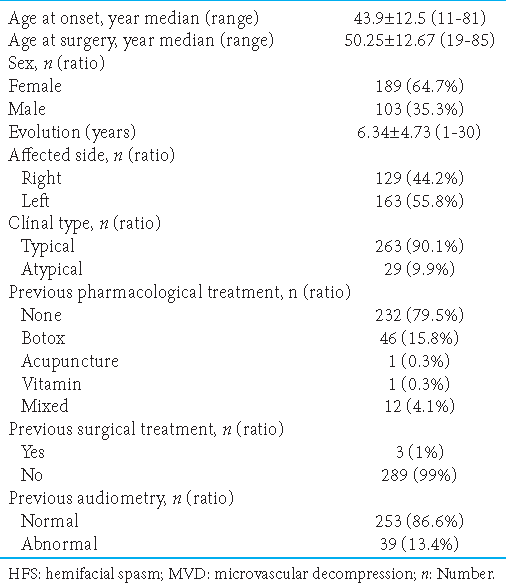
From May 1992 to September 2022, in the Neurosurgery Department of the National Institute of Neurology and Neurosurgery, we collected 292 patients diagnosed with HFS. A total of 331 MVD were made by microasterional approach without retractors. HFS occurred in 189 (64.7%) women and only 103 (35.3%) men, with a mean age of symptom onset of 43.9 ± 12.5 (range, 11–81) years and a mean evolution of 6.34 ± 4.70 (range, 1–30) years, the mean age was 50.2 ± 12.6 (range, 19–85) years. The left side was the most frequently affected at 55.8%, while the right was 44.2%. About 90.1% had a typical HFS presentation and only 9.9% were atypical [
Preoperative findings
In 174 patients (59.6%), the neurological examination was regular; however, in some cases, 14 patients (4.8%), HFS was present together with some other alterations, in with ipsilateral trigeminal neuralgia (painful convulsive tic), and in 28 patients (9.6%) with vestibulocochlear alterations, respectively. The treatment received by 46 patients (15.8%) was botox as monotherapy. Other treatments used were acupuncture and multivitamins (0.3%). All patients underwent a previous magnetic resonance study. Vascular compression was evident in 171 patients (58.6%). A previous PTA was performed within the surgical protocol, where 39 patients (13.4%) showed an abnormal development; the rest were regular [
Surgical findings, postoperative complications, and follow-up of patients who underwent MVD for HFS
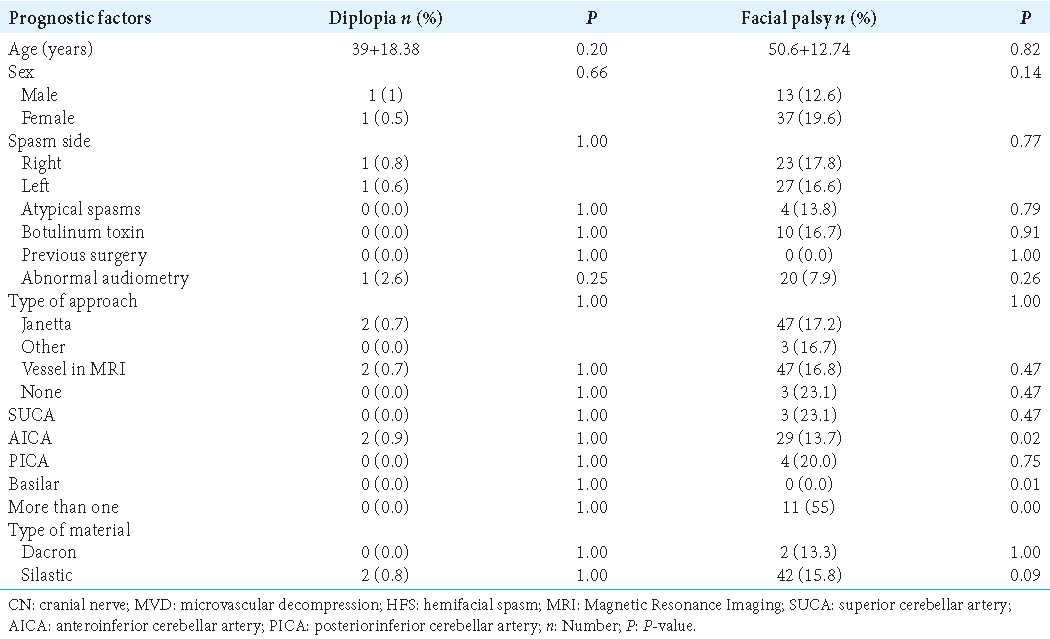
The main complication was peripheral facial paralysis in 50 patients (17.1%), being of a transitory nature the most common in 41 patients (82%). The second most frequent complication was hypoacusis in 22 patients (7.5%), which remitted in 14 of them (63%). The third most frequent complication was ipsilateral deafness in 17 patients (5.8%), associated with a failed surgical outcome (P = 0.0002). Complete remission was obtained in the nine patients who presented vertigo. Of the 12 patients who presented tinnitus, all obtained improvement in the follow-up; nine of them with complete remission. The average follow-up was 82.64 ± 37.9 months (range 1–281). A total of 256 patients (87.7%) were considered “cured,” with excellent postoperative and follow-up results. On the other hand, 36 patients (12.3%) were categorized as “failed” defined by partial postoperative development or recurrence. Of these 36 failed patients, 33 achieved a complete cure of HFS after reoperation (29 in the second and four in the third steps), two improved at follow-up, and one definitive failure [
VI and VII cranial nerve-related complications
Diplopia and facial palsy
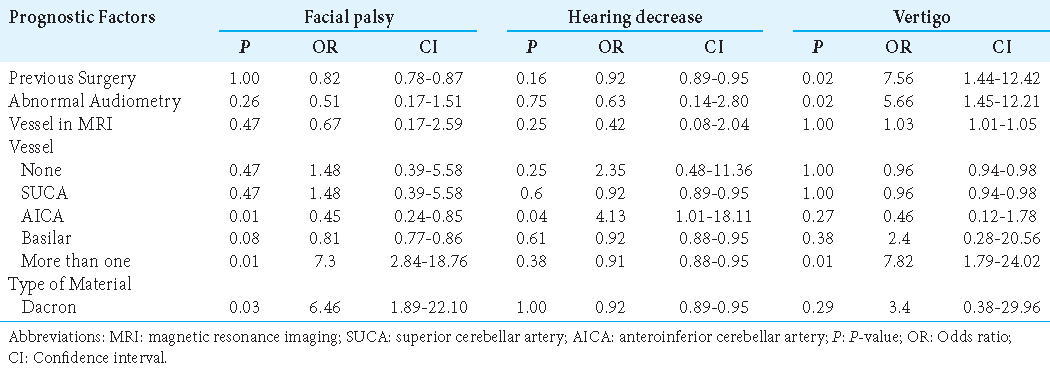
Of the 50 patients who presented with postoperative facial paralysis, 40 (80%) had a satisfactory recovery during the first 6 months of follow-up. The anterior inferior cerebellar artery (AICA) was an offending vessel and the involvement of more than one vessel was significantly associated with the development of diplopia and facial palsy (P = 0.01). We did not recognize prognostic factors associated with cranial nerve VI complications [
VIII cranial nerve-related complications
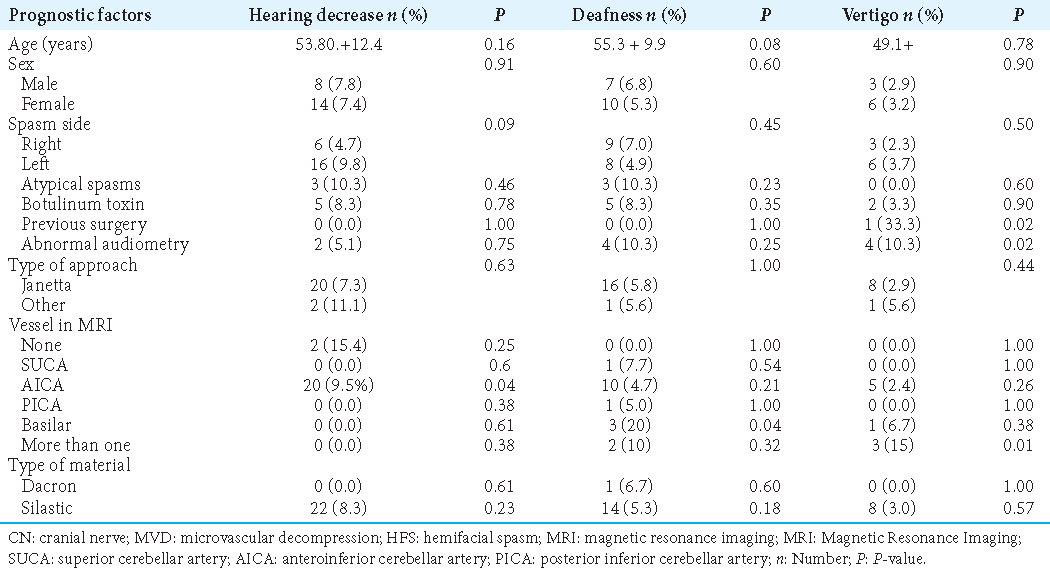
Hearing decrease, deafness, and vertigo
To describe the auditory function, we used the American Academy of Otolaryngology-Head and Neck Surgery classification, published in 1995.[
Others
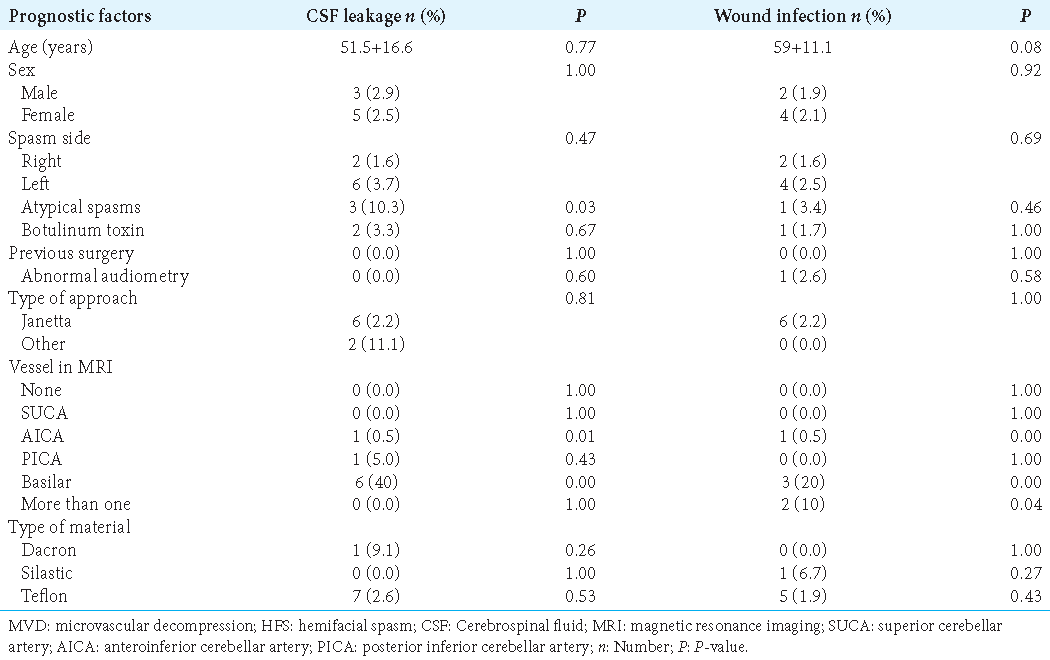
Cerebrospinal fluid (CSF) leakage and wound infection
As complications, CSF leakage occurred in seven patients (2.3%) and wound infection in 6 patients (2.1%); all cases resolved within 2 weeks. The presence of a CSF leak was associated with a history of atypical spasm preoperatively (P = 0.03), as was the involvement of the AICA as the offending vessel (P = 0.01). In addition, the risk of postoperative wound infection increased when the offending vessels were more than one (P = 0.04) (odds ratio = 7.4, 1.2–13.4) [
DISCUSSION
MVD is a functional surgery that has proven to be a definitive treatment for HFS. Surgical planning should be individualized by considering predictive variables and the potential risks in each patient. Although, in our experience, good to excellent surgical outcomes are obtained without INM, there is global advocacy in the use of INM, all measuring the clinical benefit according to the reduction in the number of cramps postoperatively. Although severe from a functional perspective, its complications are reported in <10% of all cases, with a mortality of 0.1% reported in the literature.[
We must take into account that all surgeries were made by skilled neurosurgeons in MVD, and, therefore, variability in surgical outcomes secondary to surgeon competence can be ruled out in this series. Wei et al. performed an analysis of MVD without INM and they concluded that monitoring does not appear to provide significant benefit with respect to the outcome.[
Neurophysiological monitoring
The INM objective during MVD for HFS is to prevent intraoperative injury to neural structures such as the vestibulocochlear nerve, which is directly adjacent to the facial nerve; INM can additionally assess and optimize vascular decompression and identify the offending vessel to improve the accuracy of the decompression procedure.[
VI cranial nerve cranial nerve-related complications
Trochlear nerve affection is rarely reported,[
VII cranial nerve-related complications
Facial palsy is one of the most frequent neurological complications of HFS. It can be classified as immediate or delayed and has been reported by Huh et al. to have a statistically significant association regarding onset and severity, being the more rapid and severe facial palsies.[
VIII cranial nerve-related complications
Along with facial palsy, auditory impairment is one of the most frequent neurological complications of MVD. The estimated occurrence reported in the previous studies varies from 1.9 to 20%, with reports varying in the degrees of affection and functional status evaluations due to the lack of consensus in the presurgical evaluation. Although permanent deficits are rarely presented and are associated with older age, the literature suggests an underestimation of hearing loss secondary to MVD due to a lack of consensus regarding diagnostic criteria for this complication after surgery and the absence of an appropriate pre- and postoperative auditory evaluation.[
Other complications
CSF leakage is reported in the literature as one of the most common complications following MVD, though possibly overestimated because it is a complication that tends to reduce its incidence as the learning curve stabilizes and that can often be misdiagnosed.[
CONCLUSION
MVD is the method with the highest long-term cure rates for treating HFS. However, we must inquire into the multiple factors of the patient and the surgeon to predict surgical outcomes. INM is not a must during MVD for HFS; for surgeons with less experience, INM could be helpful until they reach an optimal level in their learning curve, although it is known that INM is not available in all neurosurgical centers, we recommend its use whenever possible but its use should not be a rule of thumb. This procedure should adapt to socioeconomic circumstances. We think that MVD should not be delayed in centers that do not have intraoperative neuromonitoring with brainstem auditory evoked potentials, but the increased risk of PA should be taken into account when the patient undergoes surgical reintervention, where it could be more useful. Presenting normal audiometry and left-sided HFS are of better prognosis.
Declaration of patient consent
Patients’ consent not required as patients’ identities were not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The author(s) confirms that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Abbruzzese G, Berardelli A, Defazio G. Hemifacial spasm. Handb Clin Neurol. 2011. 100: 675-80
2. Barker FG, Jannetta PJ, Bissonette DJ, Shields PT, Larkins MV, Jho HD. Microvascular decompression for hemifacial spasm. J Neurosurg. 1995. 82: 201-10
3. Bartindale M, Mohamed A, Bell J, Kircher M, Hill J, Anderson D. Neurotologic complications following microvascular decompression: A retrospective study. J Neurol Surg B Skull Base. 2020. 81: 37-42
4. Chaudhry N, Srivastava A, Joshi L. Hemifacial spasm: The past, present and future. J Neurol Sci. 2015. 356: 27-31
5. Committee on Hearing and Equilibrium guidelines for the evaluation of hearing preservation in acoustic neuroma (vestibular schwannom. American Academy of Otolaryngology-head and Neck Surgery Foundation, INC. Otolaryngol Head Neck Surg. 1995. 113: 179-80
6. Dannenbaum M, Lega BC, Suki D, Harper RL, Yoshor D. Microvascular decompression for hemifacial spasm: Long-term results from 114 operations performed without neurophysiological monitoring. J Neurosurg. 2008. 109: 410-5
7. House JW, Brackmann DE. Facial nerve grading system. Otolaryngol Head Neck Surg. 1985. 93: 146-7
8. Hua Z, Da TY, Hui WX, Tingting Y, Jin Z, Yan Y. Delayed facial palsy after microvascular decompression for hemifacial spasm. J Craniofac Surg. 2016. 27: 781-3
9. Huh R, Han IB, Moon JY, Chang JW, Chung SS. Microvascular decompression for hemifacial spasm: Analyses of operative complications in 1582 consecutive patients. Surg Neurol. 2008. 69: 153-7 discussion 157
10. Jung NY, Lee SW, Park CK, Chang WS, Jung HH, Chang JW. Hearing outcome following microvascular decompression for hemifacial spasm: Series of 1434 cases. World Neurosurg. 2017. 108: 566-71
11. Lee JM, Park HR, Choi YD, Kim SM, Jeon B, Kim HJ. Delayed facial palsy after microvascular decompression for hemifacial spasm: Friend or foe?. J Neurosurg. 2018. 129: 299-307
12. Lee MH, Jee TK, Lee JA, Park K. Postoperative complications of microvascular decompression for hemifacial spasm: Lessons from experience of 2040 cases. Neurosurg Rev. 2016. 39: 151-8 discussion 158
13. Li F, Liu R. Clinical analysis of microvascular decompression in patients with hemifacial spasm: A retrospective study. Ann Palliat Med. 2020. 9: 318-23
14. Li N, Zhao WG, Pu CH, Yang WL. Correlation between cerebellar retraction and hearing loss after microvascular decompression for hemifacial spasm: A prospective study. World Neurosurg. 2017. 102: 97-101
15. Miller LE, Miller VM. Safety and effectiveness of microvascular decompression for treatment of hemifacial spasm: A systematic review. Br J Neurosurg. 2012. 26: 438-44
16. Park SK, Joo BE, Park K. Intraoperative neurophysiological monitoring during microvascular decompression surgery for hemifacial spasm. J Korean Neurosurg Soc. 2019. 62: 367-75
17. Rhee DJ, Kong DS, Park K, Lee JA. Frequency and prognosis of delayed facial palsy after microvascular decompression for hemifacial spasm. Acta Neurochir (Wien). 2006. 148: 839-43 discussion 843
18. Sindou M, Mercier P. Microvascular decompression for hemifacial spasm: Surgical techniques and intraoperative monitoring. Neurochirurgie. 2018. 64: 133-43
19. Sindou M, Mercier P. Microvascular decompression for hemifacial spasm: Outcome on spasm and complications. A review. Neurochirurgie. 2018. 64: 106-16
20. Sindou MP. Microvascular decompression for primary hemifacial spasm. Importance of intraoperative neurophysiological monitoring. Acta Neurochir (Wien). 2005. 147: 1019-26 discussion 1026
21. Soriano-Baron H, Vales-Hidalgo O, Arvizu-Saldana E, Moreno-Jimenez S, Revuelta-Gutierrez R. Hemifacial spasm: 20-year surgical experience, lesson learned. Surg Neurol Int. 2015. 6: 83
22. Wei Y, Yang W, Zhao W, Pu C, Li N, Cai Y. Microvascular decompression for hemifacial spasm: Can intraoperative lateral spread response monitoring improve surgical efficacy?. J Neurosurg. 2018. 128: 885-90
23. Yaltho TC, Jankovic J. The many faces of hemifacial spasm: Differential diagnosis of unilateral facial spasms. Mov Disord. 2011. 26: 1582-92