- Department of Medical Education, Dr. Kiran C. Patel College of Allopathic Medicine, Nova Southeastern University, Fort Lauderdale, United States.
- Department of Neurosurgery, Tulane Center for Clinical Neurosciences, Tulane University School of Medicine, New Orleans, United States.
Correspondence Address:
Mohammadali M. Shoja, MD Department of Medical Education, Dr. Kiran C. Patel College of Allopathic Medicine, Nova Southeastern University, Fort Lauderdale, USA.
DOI:10.25259/SNI_962_2023
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Tara Tritsch1, Mohammadali M. Shoja1, R. Isaiah Tubbs2, R. Shane Tubbs2. Middle meningeal artery arising from the petrous internal carotid artery: Outcome of unusual stapedial artery regression. 23-Feb-2024;15:59
How to cite this URL: Tara Tritsch1, Mohammadali M. Shoja1, R. Isaiah Tubbs2, R. Shane Tubbs2. Middle meningeal artery arising from the petrous internal carotid artery: Outcome of unusual stapedial artery regression. 23-Feb-2024;15:59. Available from: https://surgicalneurologyint.com/surgicalint-articles/12761/
Abstract
Background: The internal and external carotid arterial systems are generally separate regarding branching patterns. However, these two systems do form collateral circulations with their terminal parts. On rare occasions, branches that belong to one arterial system may arise from the other.
Case Description: We present a rare variant of a middle meningeal artery, generally derived from the external carotid artery, arising from the internal carotid artery and entering the floor of the middle cranial fossa by traveling through a small unnamed foramen. This anatomy and embryology and other variants of the middle meningeal and petrous carotid systems are discussed.
Conclusion: Embryologically, this variant anatomy signifies an atypical regression of the distal stapedial artery and its connection to the external carotid artery. Surgeons who operate on the skull base, vascular interventionalists, and radiologists should be aware of this potential anatomical variation of the skull base.
Keywords: Anatomy, Artery, Carotid, Meninges, Temporal bone
INTRODUCTION
The middle meningeal artery typically originates from the maxillary artery, a branch of the external carotid artery, within the infratemporal fossa. It then passes superiorly behind the mandibular nerve to enter the middle cranial fossa through the foramen spinosum.[
Anatomical variations of the middle meningeal artery may encompass its origin, branches, and potential anastomoses.[
CASE REPORT
During the routine dissection of a 78-year-old male cadaver, an unusual source of the middle meningeal artery was noted. The variation was found on the left side only, and no other intracranial variations were noted. The donor died from pneumonia. The dura mater was cut circumferentially after removing the calvaria with a bone saw. The brain was removed. Laterally, the distribution of the middle meningeal artery was typical. However, medially, the vessel’s origin was from the petrous portion of the ICA [
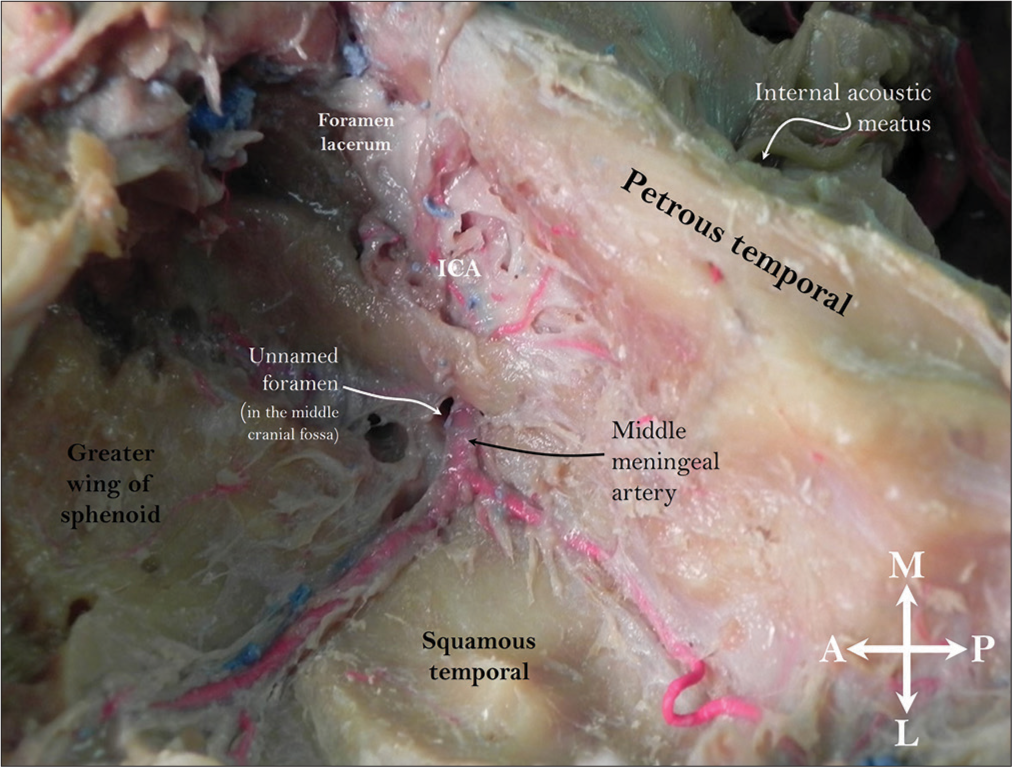
Figure 1:
Left middle cranial fossa illustrating the arterial variant identified in this case report. Note the petrous internal carotid artery giving rise to the middle meningeal artery. Observe the unnamed foramen at the skull base that the artery uses to gain access from the petrous carotid artery to the floor of the middle cranial fossa. ICA: Internal carotid artery.
DISCUSSION
The ICA comprises cervical, petrous, cavernous, and supraclinoid segments.[
Variations
Variations of the middle meningeal artery should be known to the neurosurgeon and those who interpret cranium imaging. Several arterial variants of this vessel have been reported.[
Omeis et al. reported a patient with multiple intracranial vascular anomalies, including left temporal lobe cavernoma and cavernous angioma.[
Embryology
Tandler’s seminal work offers profound insights into the intricate embryonic development of the cranial arterial system [
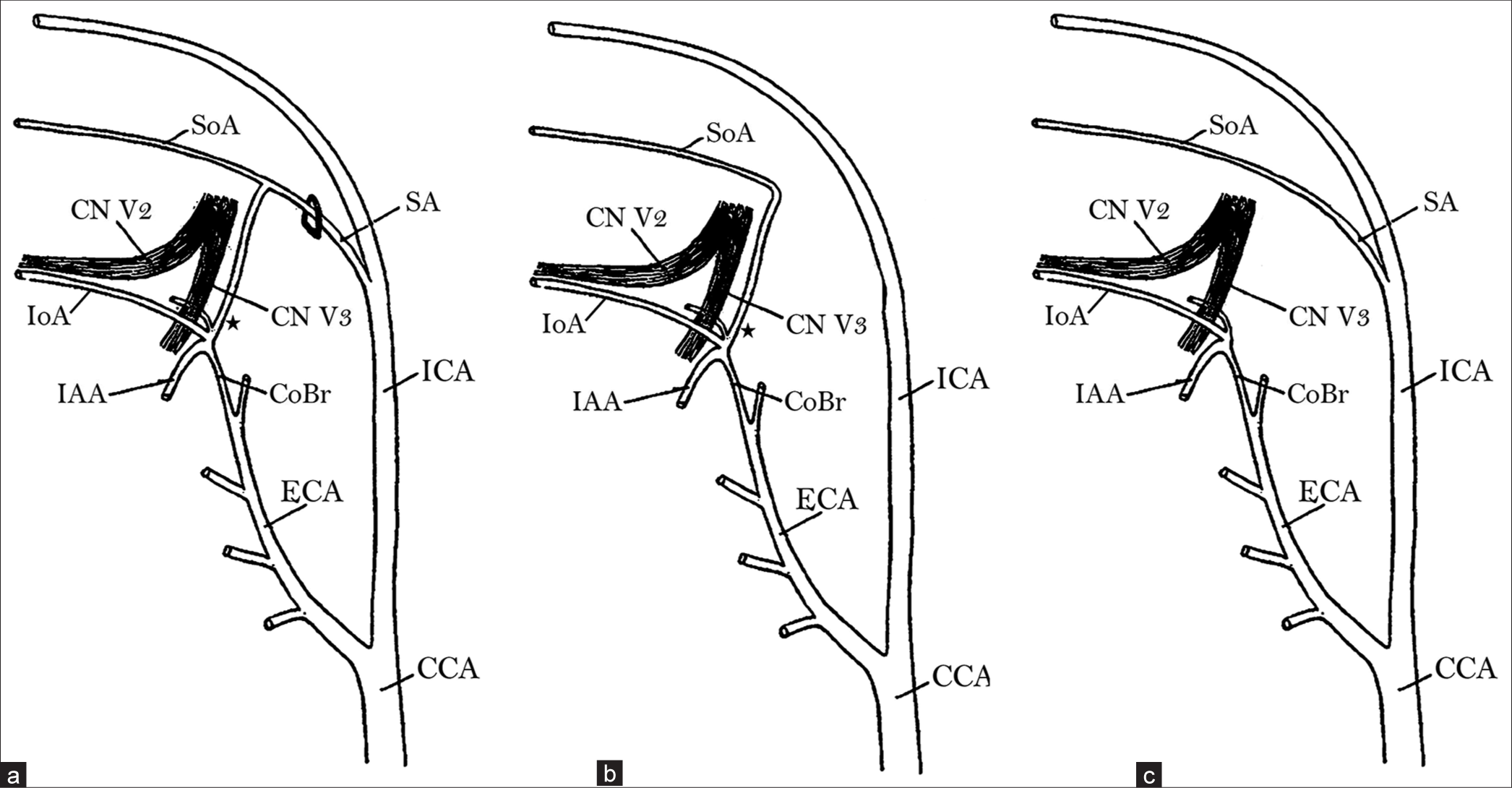
Figure 2:
Embryogenesis of the middle meningeal artery and its variants. Stapedial artery and its communication (asterisk) with the external carotid artery in a 19-mm embryo (adopted from Tandler, 1902). (a) Shows an intact stapedial artery in an early developmental stage. (b) Shows typical regression of the proximal portion of the stapedial artery, leaving the SoA (the future middle meningeal artery) connected to the maxillary artery. (c) Shows atypical regression of the distal portion of the stapedial artery, leaving the SoA connected to the internal carotid artery. CCA: Common carotid artery; CoBr: Communicating branch; CN V2: Cranial nerve V2 (maxillary nerve); CN V3: Cranial nerve V3 (mandibular nerve); ECA: External carotid artery; IAA: Inferior alveolar artery; ICA: Internal carotid artery; IoA: Infraorbital artery; SA: Stapedial artery; SoA: Supraorbital artery.
CONCLUSION
The present case highlights an exceedingly rare occurrence in which the middle meningeal artery originates from the petrous part of the ICA and enters the floor of the middle cranial fossa by traveling through a small unnamed foramen. Embryologically, this variant anatomy signifies an anomalous regression of the stapedial artery and its communicating branch to the external carotid artery. In typical development, the proximal portion of the stapedial artery regresses. While the supraorbital ramus of the stapedial artery gives rise to the middle meningeal artery, the remaining distal segment of the stapedial artery maintains its connection with the external carotid arterial system through the maxillary artery. However, in the observed case, this developmental pattern is reversed, whereby the distal portion of the stapedial artery, instead of the proximal portion, undergoes regression. This unique scenario results in the middle meningeal artery being directly connected to the ICA. Although uncommon, arterial variants described in this case report should be remembered by neurosurgeons who treat skull base lesions and radiologists who interpret imaging or perform minimally invasive procedures in this region.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that they have used artificial intelligence (AI) technology for assisting in language editing of the manuscript only.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgment
The authors wish to sincerely thank those who donated their bodies to science so that anatomical research could be performed. Results from such research can potentially improve patient care and increase mankind’s overall knowledge. Therefore, these donors and their families deserve our highest gratitude. During the preparation of this work, the authors used artificial intelligence (GPT 3.5) to improve readability and language. After using this tool/service, the authors reviewed and edited the content as needed and take(s) full responsibility for the content of the publication. No part or scientific content within this manuscript has been generated by artificial intelligence (AI).
References
1. Altmann F. Anomalies of the internal carotid artery and its branches; their embryological and comparative anatomical significance; report of a new case of persistent stapedial artery in man. Laryngoscope. 1974. 57: 313-39
2. Baltsavias G, Kumar R, Valavanis A. The pharyngo-tympanostapedial variant of the middle meningeal artery: A case report. Interv Neuroradiol. 2012. 18: 255-8
3. Bergman R, Thompson S, Afifi A, Saadeh F, editors. Compendium of human anatomic variation: Catalog, atlas and world literature. Munich: Urban and Schwarzenberg; 1988. p.
4. Bonasia S, Smajda S, Ciccio G, Robert T. Middle meningeal artery: Anatomy and variations. AJNR Am J Neuroradiol. 2020. 41: 1777-85
5. Bouthillier A, Van Loveren HR, Keller JT. Segments of the internal carotid artery: A new classification. Neurosurgery. 1996. 38: 425-33
6. Chandler S, Derezinski C. The variations of the middle meningeal artery within the middle cranial fossa. Anat Rec. 1935. 62: 309-19
7. Cironi K, Wang C, Iwanaga J, Lockwood J, Mathkour M, Bui CJ. Anatomical study of the internal carotid venous plexus: New findings with application to skull base surgery. Acta Neurochir (Wien). 2022. 164: 1923-8
8. Da Silva T, Ellwanger J, Rosa H, De Campos D. Origins of the middle meningeal artery and its probable embryological mechanism. J Morphol Sci. 2013. 30: 69-72
9. Freckmann N, Sartor K, Herrmann H. Traumatic arteriovenous fistulae of the middle meningeal artery and neighbouring veins or Dural sinuses. Acta Neurochir (Wien). 1981. 55: 273-81
10. House H, Patterson M. Persistent stapedial artery: Report of two cases. Trans Am Acad Ophthalmol Otolaryngol. 1964. 68: 644-6
11. Ironside N, Nguyen C, Do Q, Ugiliweneza B, Chen CJ, Sieg EP. Middle meningeal artery embolization for chronic subdural hematoma: A systematic review and meta-analysis. J Neurointerv Surg. 2021. 13: 951-7
12. Kimball D, Kimball H, Shane Tubbs R, Loukas M. Variant middle meningeal artery origin from the ophthalmic artery: A case report. Surg Radiol Anat. 2015. 37: 105-8
13. Kuruvilla A, Aguwa AN, Lee AW, Xavier AR. Anomalous origin of the middle meningeal artery from the posterior inferior cerebellar artery. J Neuroimaging. 2011. 21: 269-72
14. LoVerde ZJ, Shlapak DP, Benson JC, Carlson ML, Lane JI. The many faces of persistent stapedial artery: CT findings and embryologic explanations. AJNR Am J Neuroradiol. 2021. 42: 160-6
15. Manjunath K. Anomalous origin of the middle meningeal artery: A review. J Anat Soc India. 2001. 50: 179-83
16. McLennan JE, Rosenbaum AE, Haughton VM. Internal carotid origins of the middle meningeal artery: The ophthalmic-middle meningeal and stapedial-middle meningeal arteries. Neuroradiology. 1974. 7: 265-75
17. Omeis I, Crupain M, Tenner M, Murali R. Anomalous origin of the middle meningeal artery from the petrous segment of the internal carotid artery associated with multiple cerebrovascular abnormalities. Internet J Radiol. 2005. 4: 1-5
18. Rodesch G, Choi IS, Lasjaunias P. Complete persistence of the hyoido-stapedial artery in man-case report: Intra-petrous origin of the maxillary artery from ICA. Surg Radiol Anat. 1991. 13: 63-5
19. Royle G, Motson R. An anomalous origin of the middle meningeal artery. J Neurol Neurosurg Psychiatry. 1973. 36: 874-6
20. Salgado-Lopez L, Perry A, Graffeo CS, Carlstrom LP, Leonel LC, Driscoll CL. Anatomical step-by-step dissection of complex skull base approaches for trainees: Surgical anatomy of the middle fossa approaches and anterior petrosectomy, surgical principles, and illustrative cases. J Neurol Surg B Skull Base. 2022. 83: E232-43
21. Tandler J. Zur entwicklungsgeschichte der kopfarterien bei den mammalia. Morph Jahrb. 1902. 20: 275-373
22. Tubbs RS, Hansasuta A, Loukas M, Louis RG, Shoja MM, Salter EG. Branches of the petrous and cavernous segments of the internal carotid artery. Clin Anat. 2007. 20: 596-601
23. Tubbs RS, Verm K, Riech S, Mortazavi MM, Shoja MM, Loukas M. Persistent fetal intracranial arteries: A comprehensive review of anatomical and clinical significance: A review. J Neurosurg. 2011. 114: 1127-34
24. Tunali S, Tubbs R, Shoja M, Loukas M, editors. External carotid artery. Bergman’s comprehensive encyclopedia of human anatomical variations. Hoboken: Wiley; 2016. p. 477-86
25. White HJ, Reddy V, Mesfin FB, editors. Anatomy, head and neck: Foramen spinosum. StatPearls. Treasure Island, FL: StatPearls Publishing; 2023. p. Available from: https://www.ncbi.nlm.nih.gov/books/NBK535432 [Last accessed on 2023 December 30]