- Department of Neurological Surgery, Lebanese University, Faculty of Medical Sciences,
- Department of Neurological Surgery, Neuroscience Research Center, Faculty of Medical Sciences, Lebanese University, Lebanon,
- Department of Oncology, Cleveland Clinic, Weston, Florida, United States,
- Department of Neurological Surgery, Al Rassoul Al-Azam Hospital,
- Head of Radiology Department, Zahraa Hospital University Medical Center,
- Department of Nuclear Medicine Radiobiology Radiopathology, Faculty of Medical Sciences, Lebanese University,
- Radiology Department, Bahman Hospital, Faculty of Medicine, Lebanese University,
- Head of Department of Radiology, Faculty of Medicine, Lebanese University, Bahman Hospital, Centre Hospitalier Vallee de la Maurienne-France, Beirut, Lebanon.
Correspondence Address:
Ali Msheik, Department of Neurological Surgery, Lebanese University, Faculty of Medical Sciences, Beirut, Lebanon.
DOI:10.25259/SNI_1096_2022
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ali Msheik1, Youssef Fares2, Mohammad Mohanna3, Ahmad Aoude4, Mohamad Shkeir5, Feras Chehade6, Ali Kanj7, Assaad Mohanna8. Middle meningeal artery embolisation: The review of a new treatment for chronic subdural hematomas. 24-Feb-2023;14:66
How to cite this URL: Ali Msheik1, Youssef Fares2, Mohammad Mohanna3, Ahmad Aoude4, Mohamad Shkeir5, Feras Chehade6, Ali Kanj7, Assaad Mohanna8. Middle meningeal artery embolisation: The review of a new treatment for chronic subdural hematomas. 24-Feb-2023;14:66. Available from: https://surgicalneurologyint.com/surgicalint-articles/12175/
Abstract
Background: This is a literature review aiming to provide an update about the recent findings related to the efficacy of middle meningeal artery embolization (MMAE) in the treatment of chronic subdural hematomas (cSDHs), comparison with conventional therapy and deduction of the current recommendations and indications.
Methods: The literature is reviewed using a search through the PubMed index using keywords. Studies are then screened, skimmed, and thoroughly read. 32 studies fulfilled the inclusion criteria and are included in the study.
Results: Five indications for the application of MMA embolization (MMAE) are deducted from the literature. The usage as a preventive measure after surgical treatment of symptomatic cSDHs in patients with a high risk of recurrence and the usage as a standalone procedure has been the most common reasons for indication of this procedure. Rates of failures for the aforementioned indications have been 6.8% and 3.8%, respectively.
Conclusion: The safety of MMAE as a procedure is regarded as a general theme in the literature and can be considered for future applications. Usage of this procedure in clinical trials with more patient segregation and timeframe assessment relative to surgical intervention are recommendations of this literature review.
Keywords: Burr hole, Chronic subdural hematomas, Elderly population, Inflammation, Middle meningeal artery embolization, Prevention, Recurrence
INTRODUCTION
Definition of subdural hematoma
Chronic subdural hematoma (cSDH) is defined as a liquefied hematoma in the subdural space.[
Anatomy and histology
cSDHs are encapsulated flattened structures that form within the virtual dural space [
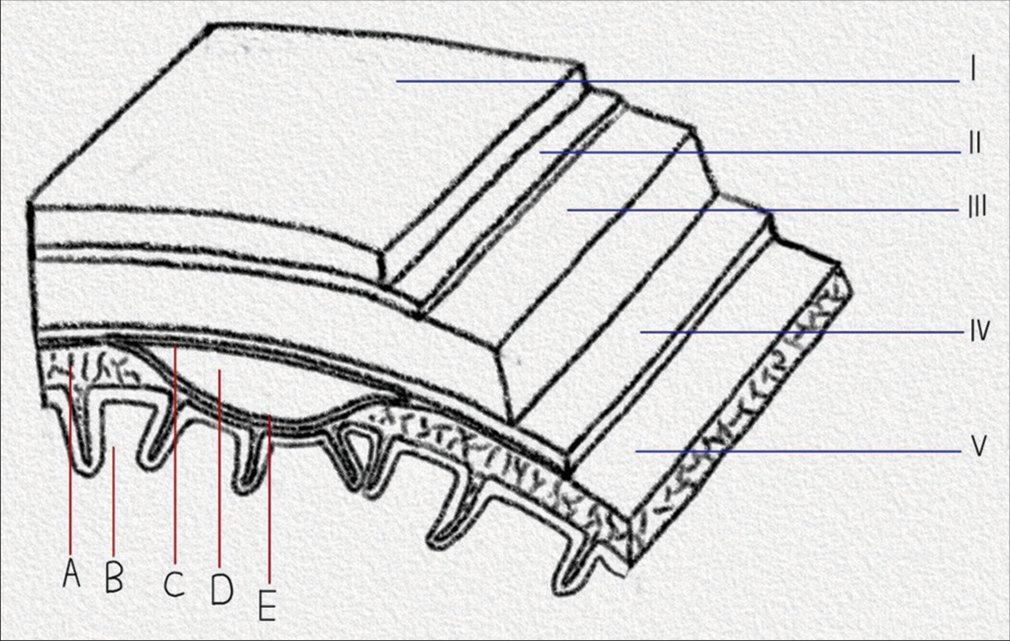
Figure 1:
Drawn presentation showing the layers from the scalp to the cerebral cortex with a space-occupying subdural hematoma. Courtesy of the author Ali Msheik. (A) The arachnoid layer of the cerebral Dural membranes in coronal view, (B) The cerebral cortex, (C) The outer layer of the chronic subdural hematoma, (D): The content of the chronic subdural hematoma, (E) The inner layer of the chronic subdural hematoma, (i) Scalp, (ii) Periosteum layer of the skull, (iii): Skull bone, (iv) Dura matter, (v) Arachnoid layer of the Dural membranes in sagittal view.
Evolution
Being of embryonic mesodermal origin, tear of the cell layer of the meninges dictates a similar response to wound healing.[
Correspondence of CT appearance with classification and prognosis
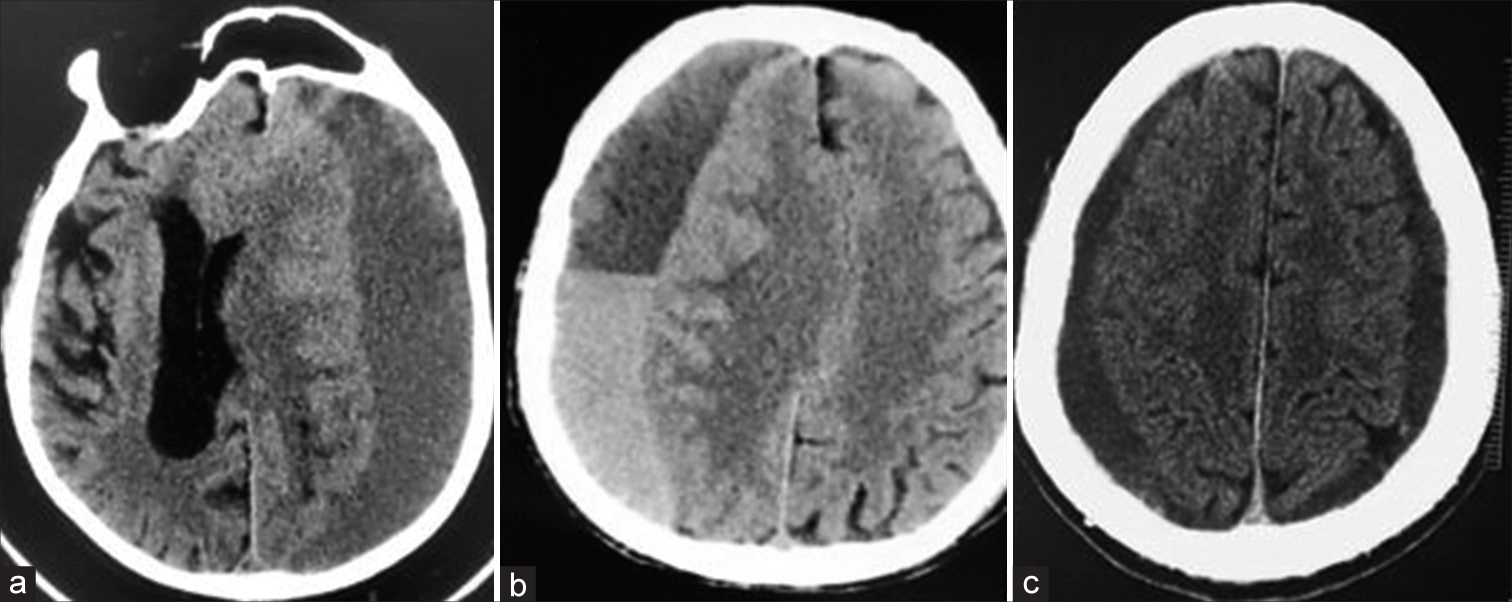
Postsurgical risk for recurrence and hematoma volume increase may be correlated to the CT scan features obtained before surgical intervention, that is, low risk is attributed to the homogenous appearance of cSDHs on CT scan.[
Causes of subdural hematoma
Bleeding
Tear of bridging veins as they traverse the dural cell layer is the most accepted cause of cSDHs.[
The transformation from hygroma
Formation of a cSDH as a progression from a subacute hygroma is another hypothesis advocated by Japanese neurosurgeons.[
Enlargement by osmotic and oncotic pressures
Indifference in osmolality and oncotic pressure between cSDH fluid, plasma, and CSF limits the credibility of the theory supporting the role of either in the enlargement of cSDHs.[
Epidemiology of cSDH
The incidence of cSDHs is not well known and varies between operated and non-operated cases. Ranging from 1.3 to 5.3/100,000/year for operative cases, the incidence naturally increases to 8.2 up to 48/3 of 17 100,000/year.[
Treatments for cSDH
Treatment of cSDH requires prior assessment of patients on an individual basis. The symptomatology of patients is key in determining the urgency and the indication for surgical intervention. Typically, patients received at the ED for trauma or acute development of neurological compromise are diagnosed or incidentally found to have cSDH.[
Correction of coagulopathy and thrombopathy
Reversal of coagulopathy instilled by OACs and antiplatelet medications, liver insufficiency, and other coagulation disease is essential for the prevention of further expansion of cSDHs and to allow control of blood loss after surgical evacuation of symptomatic cSDHs.[
Adjuvant treatments
Steroids and anti-epileptic medications are two main medical therapies adjuvant to surgical evacuation used in managing symptomatic cSDHs. In 2021 lesser recurrence of cSDH under the treatment with steroids were reported.[
The authors noted a lack of evidence of a significant reduction in the incidence of seizures in patients with cSDHs following the administration of anti-epileptic drugs.[
Conservative treatment
Conservative treatment is offered to patients whose morbid conditions hinder surgical intervention or to whom surgery will not add benefit compared to medical treatment. Patients with small asymptomatic cSDHs are managed conservatively.[
Surgical intervention
Symptomatic patients are ideal for treatment with surgical evacuation with an 80% success rate. Accomplished with minimal surgical risk, usually patients recover fast postoperatively and show the reverse neurological compromise instilled by the expanded hematoma.[
Prognosis and complications
Complications of cSDH surgical evacuation range from the ones associated with any surgery, that is, infection, bleeding and possible tissue damage to more specific ones related to the nature of the operation, that is, continuous bleeding, re-bleeding, tension pneumocephalus, seizures, intra-parenchymal bleeding, stroke, and possible brain tissue damage.[
Mortality due to cSDHs varies according to whether surgical intervention was held or not. Without surgical evacuation, mortality rises to 33%. However, it drops to 3% within 30 days of cSDHs evacuation.[
Future research areas
New approaches
Surgical evacuation and brain decompression are the main goals of the treatment of symptomatic cSDHs. Postoperative adjuvant therapies are understudied in the literature inclusive of corticosteroids, ACE inhibitors, and tranexamic acid.[
Anatomy related to MMA embolization (MMAE)
It is believed that the MMA through its anterior and posterior branches, which supply the dural layers, can anastomose with the neovascular vessels. Hence, re-bleeding through these anastomoses can provoke the recurrence of a cSDH.[
Steps of MMAE
Embolization requires an endovascular approach through the femoral artery ipsilateral to the recurrent cSDH.[
The aim of the literature review
This literature review aims to provide an update on the recent findings related to the efficacy of MMA embolization. Answers that this review will provide connectivity to the following inquiries:
Is MMA embolization an adjunctive technique besides surgical intervention? Can MMA embolization replace surgery? In which patients? What are the indications for MMAE? How efficient is MMA embolization in the prevention of recurrence after surgical intervention and expansion of minimal cSDH not requiring surgical evacuation? What does the failure of MMA embolization to prevent the recurrence of cSDH re-bleeding suggest?
MATERIALS AND METHODS
Primary revision: Identification
We reviewed the existing literature on PubMed until August 2022, in the English language. We performed the research with combinations of the keywords “MMA,” “middle meningeal artery embolization,” “embolization,” “refractory cSDH,” “chronic subdural hematoma recurrence”, and “cSDH outer layer.” All kinds of work were included. References of the relevant studies were considered as an additional source of articles. Pure literature reviews were not included. We focused on the cSDH and the embolization of MMA as means of treatment.
Secondary revision: Screening
The titles of the sequestered articles are screened. Causes of cSDH related to acute subdural and epidural hematomas and tumor-provoked cSDHs were excluded from the study. Studies inclusive of a pure methodological description of MMAE and MMA variable anatomy were excluded from the study.
Data sequestration: Inclusion and classification
As this literature review is the work of one author, data from the included studies were thoroughly read and analyzed. Referral to other recent literature reviews aided the guidance of the author and allowed further checking. The studies were classified according to the way MMAE was utilized. Hence, the patients were classified according to the indication of MMAE:
MMAE as a standalone therapy MMAE to prevent recurrence in symptomatic cSDHs MMAE in recurrent cSDHs without second surgery MMAE in patients with recurrent cSDHs after the second surgery.
RESULTS
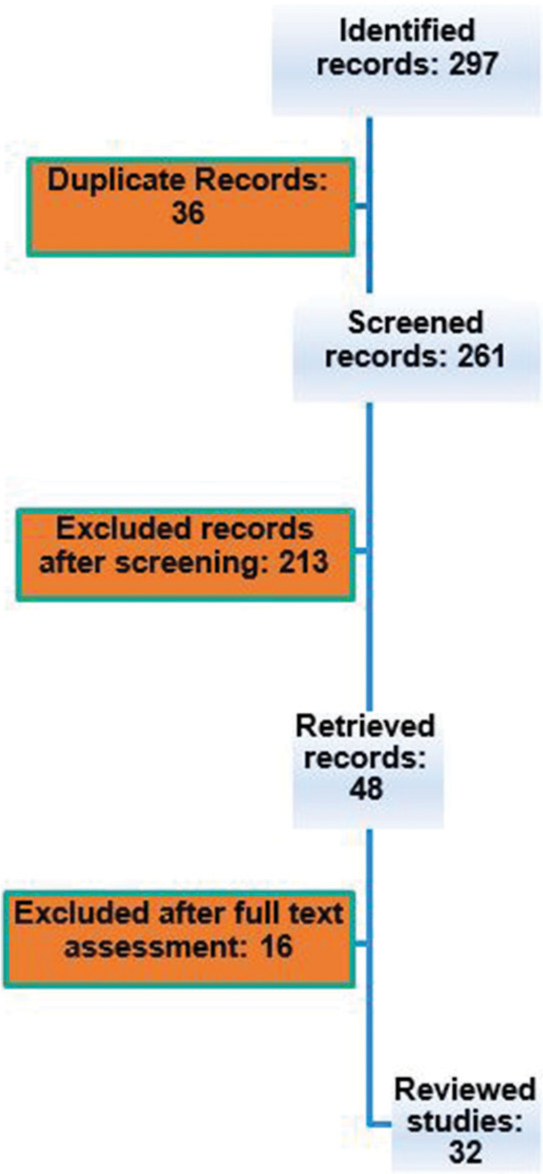
297 records were identified after a search using the combination of the aforementioned keywords on PubMed. 36 records were exempted because of being duplicate records. 213 records were excluded after screening the titles and abstracts. 16 records were excluded after reading the full text [
32 records were finally reviewed [
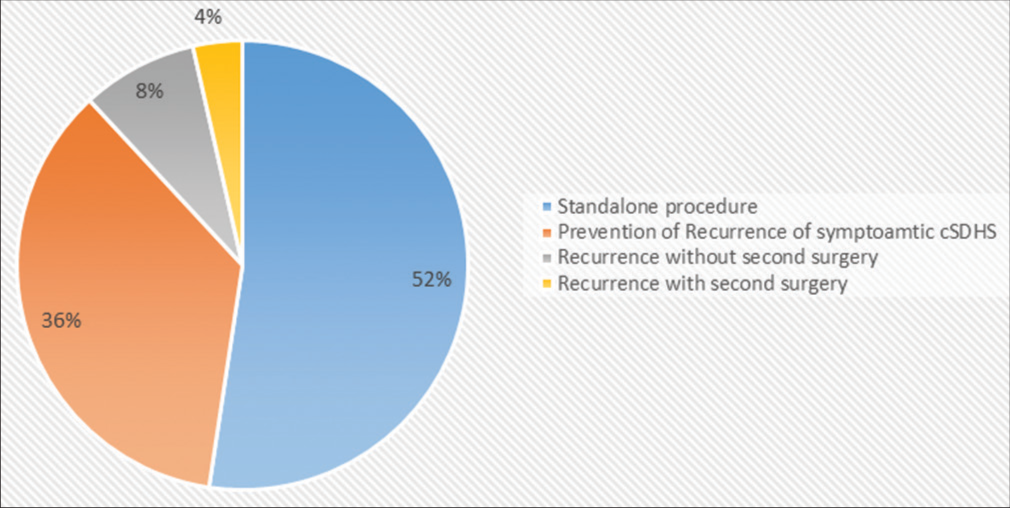
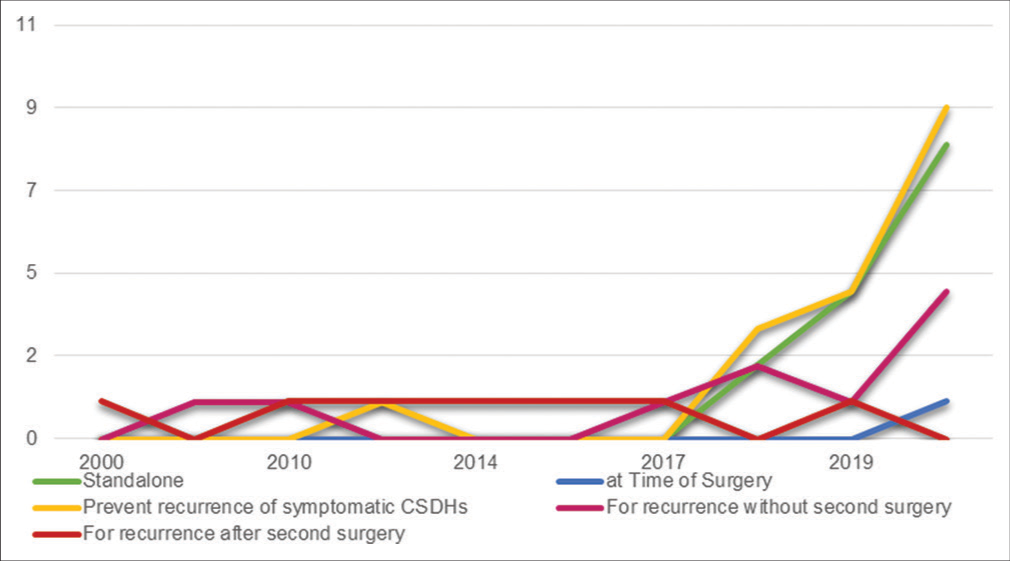
Totally, 973 patients were included and categorized into five groups. The distribution of the patients is evident in
MMAE as a standalone procedure
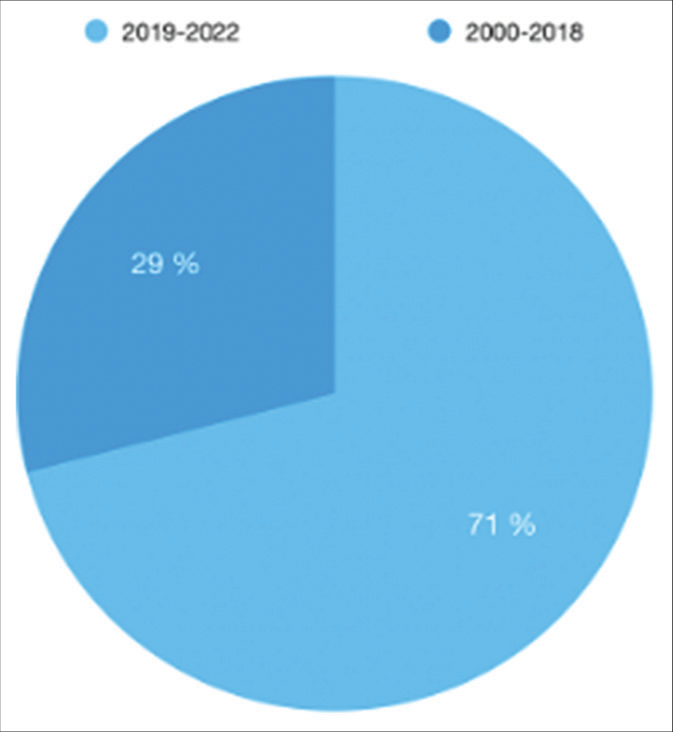
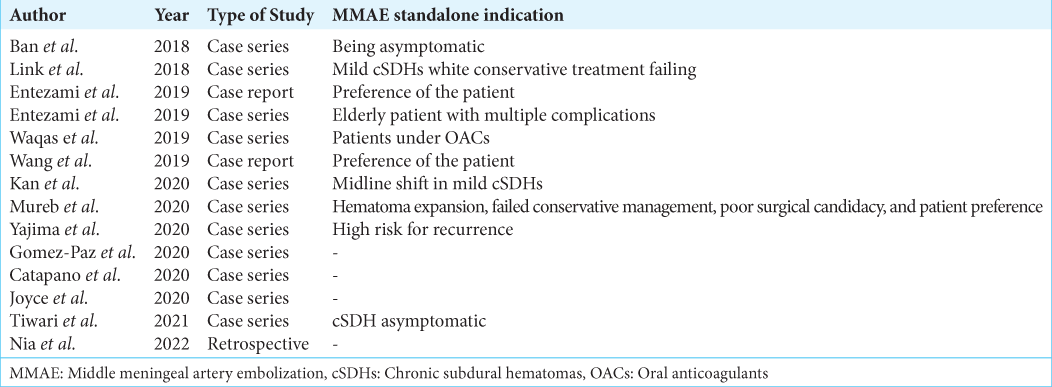
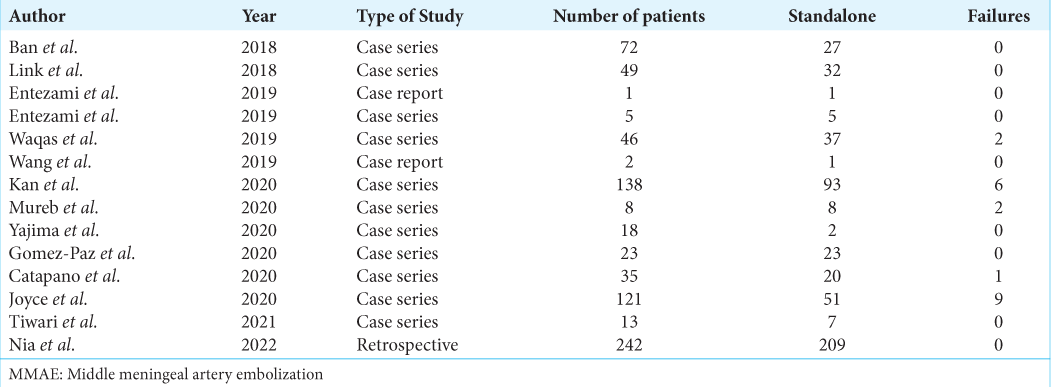
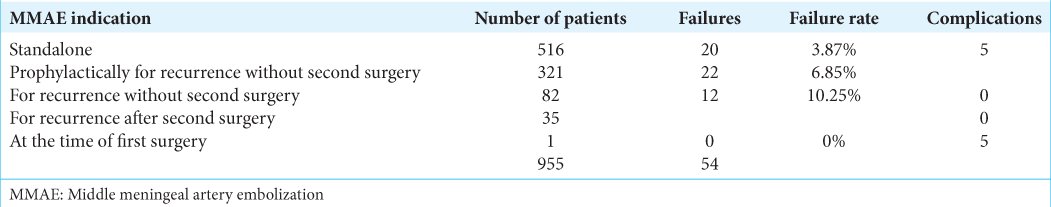
Being a standalone procedure is an application of MMAE reported in 14 of the reviewed articles published starting from the year 2018 [
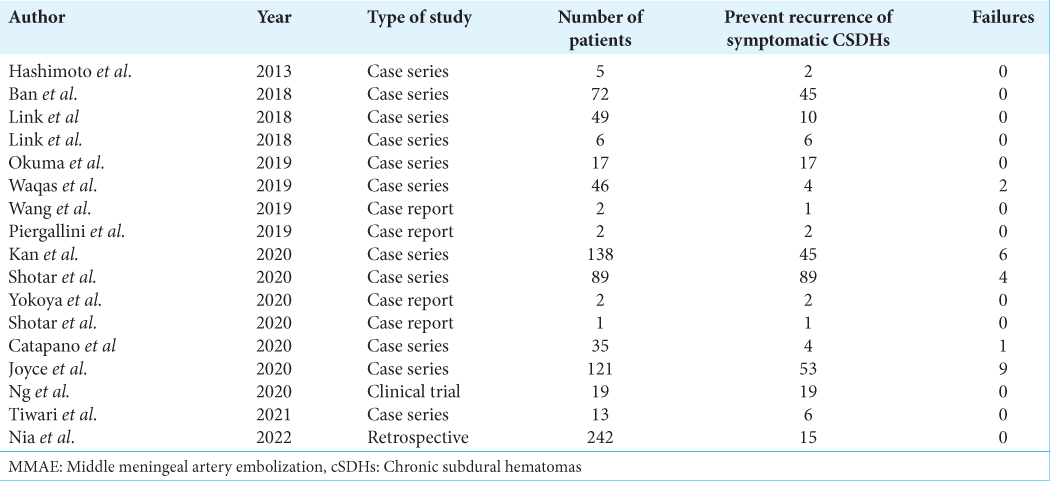
MMAE as a prevention of recurrence for symptomatic cSDHs
Of the 773 patients included in the studies, 516 (66.75%) were subject to a standalone MMAE procedure with 3.8% failure rate if compared to the total MMAE procedures done [
MMAE for recurrence of cSDHs
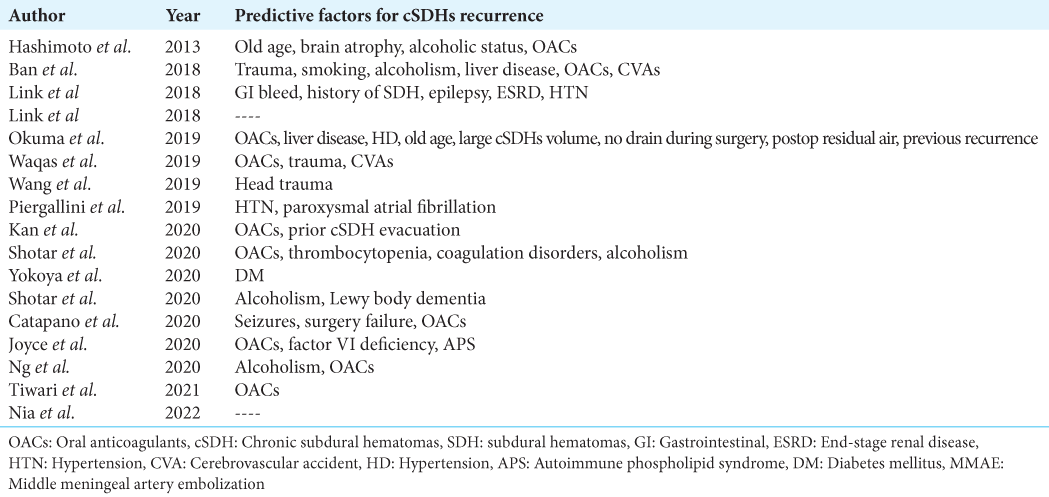
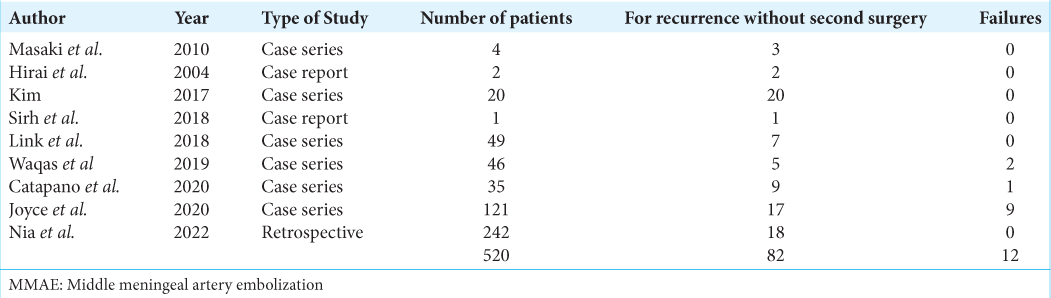
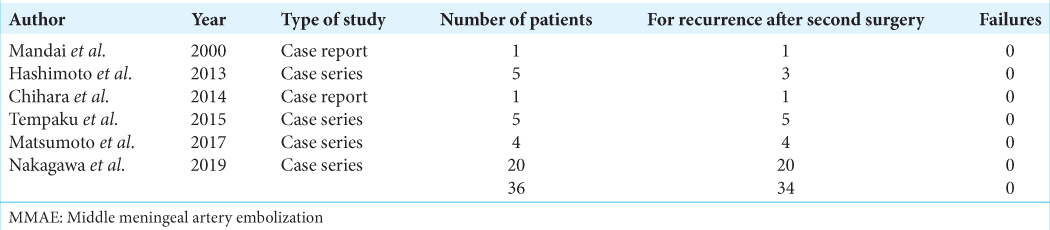
MMAE was applied for the recurrence of cSDHs either with a second surgery done or without any surgical intervention in 15 articles. Out of 556 patients included in those articles, 82 (14.74%) patients had MMAE procedures done without a second surgical intervention compared to 34 (6.29%) patients who received a second surgical intervention followed by an MMAE procedure. The only article reporting indicating MMAE for both reasons in the same study was Mino et al. in 2010.[
MMAE for recurrence without surgery
Besides Mino et al., of eight articles reporting MMAE procedures done for cSDHs recurrence without a second surgery, two were case reports, and six were case series [
MMAE after second surgery for recurrence
Besides Mino et al., only six articles reported applying MMAE procedures to patients for recurrence after the second surgery; two were case reports and four are case series [
Failure of MMAE
MMAE procedures categorized as a failure were either a procedure that has resulted in non-resorption of cSDHs, increase in the hematoma volume, or recurrence of the hematoma after resorption. Out of the 997 MMAE procedures, 22 procedures failed and 19 procedures were complicated [
Complications of MMAE
Only five complications were reported in the reviewed articles and it was noticed that all complications happened with patients whose MMAE procedures were indicated as either a standalone or a prophylactic procedure. The complications were worsening cSDHs, two CVAs, a focal seizure, and an episode of aphasia.
Comparison with conventional treatment
In the literature review published by Di Cristofori et al. in March 2022, the author reported that five studies cited a comparison between patients to whom MMAE was applied versus patients treated conventionally.[
DISCUSSION
Indications for MMA embolization through the literature
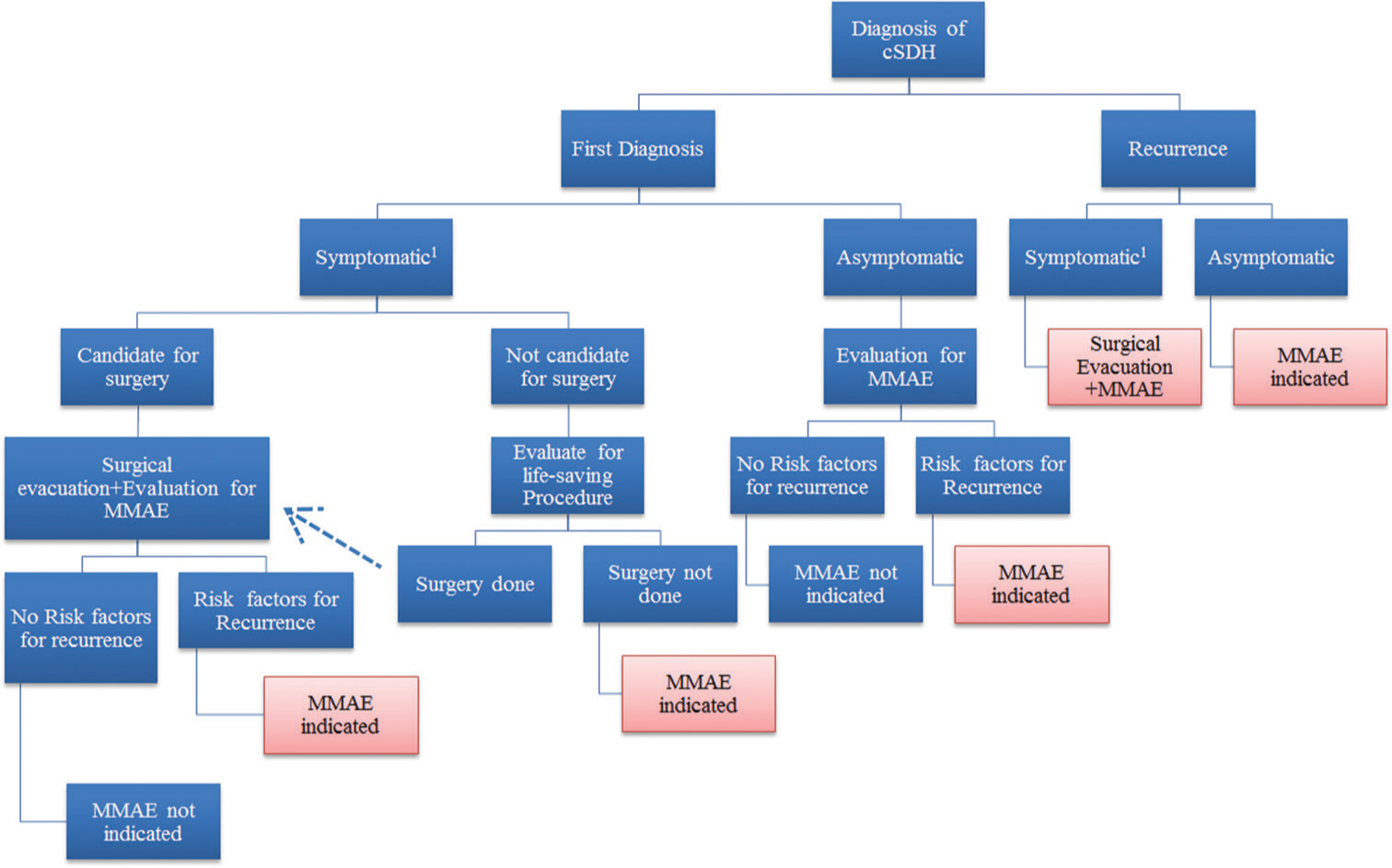
This review of literature unveiled the application of MMAE for the following indications: a standalone procedure, an adjuvant treatment at the time of first surgery, for prevention of recurrence after first surgical evacuation, as a treatment after recurrence after first surgical evacuation without second surgery and as a treatment after recurrence after second surgical evacuation. Keen attention ought to discriminate the first indication versus the other four indications. MMAE as a standalone procedure manages cSDHs by vascular compromise. Hence, further progression of cSDHs by standalone MMAE is valid. The other four scenarios target the mass effect imparted by the hematoma volume by surgical evacuation and target the vascular supply of the dural cell layer by MMAE, yet each is at a different stage regarding the surgical intervention and a number of recurrences. Therefore, this justifies the higher rate of standalone MMAE procedures failure compared to the other four modalities reported by Di Cristofori et al.[
Report of MMAE applications and results
The number of patients to whom MMAE was applied in the reviewed literature is conspicuous. Some articles reported the number of treated patients; others report the number of cSDHs yet not the number of patients. Bilateral cSDHs are reported as two cases and treatment of either has been reported by different modalities for different indications in the same individual. Limited is the report of segregation of patients according to whether the cSDHs is unilateral or bilateral. Risk factors of progression and recurrence, results of MMAE application, long-term outcomes, side effects, mortality, and morbidity need to be assessed for each of the two patient categories. This is not presented in the literature. Hence, results cannot be generalized. The heterogeneity of the data adds to the lack of parameters related to the number of patients within each category, the number of patients considered for each indication, the time between the surgical intervention and the MMAE procedure, and the type of MMAE material and technique specifications. Moreover, the categorization of patients according to morbid conditions, OACs administration and risk factor variance is missing in the articles, as a comparison among those groups is not derived.
Future considerations
Despite the aforementioned bias, discrimination between standalone MMAE and MMAE associated with surgical evacuation, regardless of the situation of either procedure, is paramount. Future application of MMAE associated with surgical evacuation is anticipated to provide better results versus standalone MMAE. This was noted previously in the results section. The mere fact that residual hematoma remains in the case of standalone MMAE constrains the efficacy of this application. This increases its failure rate as patients could fail to resorb the residual volume and re-bleeding could be a complication, especially in patients with OACs and anti-platelets intake. The necessity to surgically evacuate hematomas and consider MMAE later after surgical intervention appears as a better approach. Future consideration of studies addressing the adequate timeframe is highly recommended. This approach is anticipated to reduce the hospitalization period, and the costs of second surgical interventions, and promote a better prognosis with less morbidity and mortality, especially in the elderly population. Therefore, clinical trials inclusive of standalone MMAE applied to symptomatic yet poor surgical candidates and MMAE procedure applied at different timeframes to surgical candidates after surgical intervention versus conventional control groups are indispensable. The studies published by Nia et al. and Ng et al. are representative of the required studies.[
CONCLUSION
CSDH is a disease of the elderly who struggle with the eventual result of aging: comorbidities and chronic diseases. Treatment of this bleeding condition within the skull has been conventionally surgical. However, the recurrence of this condition and the risks implemented in the surgical evacuation of cSDHs renders less invasive procedures more appealing. Hence, the attention drives us toward MMAE. This literature review aimed to provide a walkthrough of the available data about MMAE application for the treatment of cSDHs. Anticipation of future applications of MMAE, the indications, and the foreseeable outcomes was speculated in light of the reported findings by articles over the past 22 years. As a result, application for the prevention of recurrence and as a primary modality of care has been the indication of choice. However, heterogeneity of the data limited this work and hindered the distinction between indication-specific MMAE failure rates. The safety of MMAE as a procedure is regarded as a general theme in the literature and can be considered for future application. We look forward to clinical trials with comparative themes to provide better population-specific deductions about this new technique.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Ahn JH, Jun HS, Kim JH, Oh JK, Song JH, Chang IB. Analysis of risk factor for the development of chronic subdural hematoma in patients with traumatic subdural hygroma. J Korean Neurosurg Soc. 2016. 59: 622-7
2. Altaf I, Shams S, Vohra AH. Radiological predictors of recurrence of chronic subdural hematoma. Pak J Med Sci. 2018. 34: 194-7
3. Arham A, Zaragita N. Middle meningeal artery embolization following burr hole in chronic subdural hematoma. Asian J Neurosurg. 2020. 15: 382-4
4. Asghar M, Adhiyaman V, Greenway MW, Bhowmick BK, Bates A. Chronic subdural hematoma in the elderly--a North Wales experience. J R Soc Med. 2002. 95: 290-2
5. Ban SP, Hwang G, Byoun HS, Kim T, Lee SU, Bang JS. Middle meningeal artery embolization for chronic subdural hematoma. Radiology. 2018. 286: 992-9
6. Bartek J, Sjåvik K, Dhawan S, Sagberg LM, Kristiansson K, Ståhl F. Clinical course in chronic subdural hematoma patients aged 18-49 compared to patients 50 years and above: A multicenter study and meta-analysis. Front Neurol. 2019. 10: 311
7. Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol. 2005. 7: 452-64
8. Case ME. Inflicted traumatic brain injury in infants and young children. Brain Pathol. 2008. 18: 571-82
9. Catapano JS, Nguyen CL, Wakim AA, Albuquerque FC, Ducruet AF. Middle meningeal artery embolization for chronic subdural hematoma. Front Neurol. 2020. 11: 557233
10. Chen FM, Wang K, Xu KL, Wang L, Zhan TX, Cheng F. Predictors of acute intracranial hemorrhage and recurrence of chronic subdural hematoma following burr hole drainage. BMC Neurol. 2020. 20: 92
11. Chihara H, Imamura H, Ogura T, Adachi H, Imai Y, Sakai N. Recurrence of a refractory chronic subdural hematoma after middle meningeal artery embolization that required craniotomy. NMC Case Rep J. 2014. 1: 1-5
12. Degen KE, Gourdie RG. Embryonic wound healing: A primer for engineering novel therapies for tissue repair. Birth Defects Res C Embryo Today. 2012. 96: 258-70
13. Di Cristofori A, Remida P, Patassini M, Piergallini L, Buonanno R, Bruno R. Middle meningeal artery embolization for chronic subdural hematomas. A systematic review of the literature focused on indications, technical aspects, and future possible perspectives. Surg Neurol Int. 2022. 13: 94
14. Dossani RH, Waqas M, Rai HH, Baig AA, Cappuzzo JM, Siddiqui AH. Use of N-butyl 2-cyanoacrylate (nBCA) for preoperative tumor embolization. J Neurointerv Surg. 2022. 14: neurintsurg-2021-017400
15. Edlmann E, Giorgi-Coll S, Whitfield PC, Carpenter KL, Hutchinson PJ. Pathophysiology of chronic subdural hematoma: Inflammation, angiogenesis and implications for pharmacotherapy. J Neuroinflammation. 2017. 14: 108
16. Entezami P, Field NC, Dalfino JC. Outpatient management of chronic expanding subdural hematomas with endovascular embolization to minimize inpatient admissions during the COVID-19 viral pandemic. Interv Neuroradiol. 2021. 27: 716-21
17. Entezami P, Nourollahzadeh E, Dalfino J. Embolization of middle meningeal artery for the treatment of headaches induced by chronic subdural hematoma: A case report. Headache. 2019. 59: 615-8
18. Galgano M, Toshkezi G, Qiu X, Russell T, Chin L, Zhao LR. Traumatic brain injury: Current treatment strategies and future endeavors. Cell Transplant. 2017. 26: 1118-30
19. Gardner RC, Dams-O’Connor K, Morrissey MR, Manley GT. Geriatric traumatic brain injury: Epidemiology, outcomes, knowledge gaps, and future directions. J Neurotrauma. 2018. 35: 889-906
20. Gish RG, Stravitz RT. Correction of coagulopathy of liver disease prior to procedures. Gastroenterol Hepatol (N Y). 2021. 17: 16-23
21. Gomez-Paz S, Akamatsu Y, Salem MM, EnriquezMarulanda A, Robinson TM, Ogilvy CS. Upfront middle meningeal artery embolization for treatment of chronic subdural hematomas in patients with or without midline shift. Interv Neuroradiol. 2021. 27: 571-6
22. Hamou H, Alzaiyani M, Rossmann T, Pjontek R, Kremer B, Zaytoun H. Seizure after surgical treatment of chronic subdural hematoma-associated factors and effect on outcome. Front Neurol. 2022. 13: 977329
23. Hashimoto T, Ohashi T, Watanabe D, Koyama S, Namatame H, Izawa H. Usefulness of embolization of the middle meningeal artery for refractory chronic subdural hematomas. Surg Neurol Int. 2013. 4: 104
24. Hirai S, Ono J, Odaki M, Serizawa T, Nagano O. Embolization of the middle meningeal artery for refractory chronic subdural haematoma. Usefulness for patients under anticoagulant therapy. Interv Neuroradiol. 2004. 10: 101-4
25. Ihab Z. Pneumocephalus after surgical evacuation of chronic subdural hematoma: Is it a serious complication?. Asian J Neurosurg. 2012. 7: 66-74
26. Iorio-Morin C, Blanchard J, Richer M, Mathieu D. Tranexamic Acid in Chronic Subdural Hematomas (TRACS): Study protocol for a randomized controlled trial. Trials. 2016. 17: 235
27. Jeong SI, Kim SO, Won YS, Kwon YJ, Choi CS. Clinical analysis of risk factors for recurrence in patients with chronic subdural hematoma undergoing burr hole trephination. Korean J Neurotrauma. 2014. 10: 15-21
28. Joyce E, Bounajem MT, Scoville J, Thomas AJ, Ogilvy CS, Riina HA. Middle meningeal artery embolization treatment of nonacute subdural hematomas in the elderly: A multiinstitutional experience of 151 cases. Neurosurg Focus. 2020. 49: E5
29. Kan P, Maragkos GA, Srivatsan A, Srinivasan V, Johnson J, Burkhardt JK. Middle meningeal artery embolization for chronic subdural hematoma: A multi-center experience of 154 consecutive embolizations. Neurosurgery. 2021. 88: 268-77
30. Kim E. Embolization therapy for refractory hemorrhage in patients with chronic subdural hematomas. World Neurosurg. 2017. 101: 520-7
31. Ko BS, Lee JK, Seo BR, Moon SJ, Kim JH, Kim SH. Clinical analysis of risk factors related to recurrent chronic subdural hematoma. J Korean Neurosurg Soc. 2008. 43: 11-5
32. Kwon SM, Lee MH, Seo Y, Kim YI, Oh HJ, Kim KH. A radiological assessment of chronic subdural hematomas. Korean J Neurotrauma. 2022. 18: 12-21
33. LaPelusa A, Dave HD, editors. Physiology, Hemostasis. Treasure Island (FL): StatPearls Publishing; 2022. p.
34. Laviv Y, Rappaport ZH. Risk factors for development of significant chronic subdural hematoma following conservative treatment of acute subdural hemorrhage. Br J Neurosurg. 2014. 28: 733-8
35. Lee IH, Choi JI. A retrospective study from a single center of 208 patients with unilateral chronic subdural hematoma to compare outcomes following burr hole craniotomy and hematoma drainage within 48 hours and between 48 hours and 5 days. Med Sci Monit. 2022. 28: e936774
36. Lee KS. History of chronic subdural hematoma. Korean J Neurotrauma. 2015. 11: 27-34
37. Lee KS. How to treat chronic subdural hematoma? Past and now. J Korean Neurosurg Soc. 2019. 62: 144-52
38. Li S, Nguyen IP, Urbanczyk K. Common infectious diseases of the central nervous system-clinical features and imaging characteristics. Quant Imaging Med Surg. 2020. 10: 2227-59
39. Link TW, Boddu S, Paine SM, Kamel H, Knopman J. Middle meningeal artery embolization for chronic subdural hematoma: A series of 60 cases. Neurosurgery. 2019. 85: 801-7
40. Link TW, Schwarz JT, Paine SM, Kamel H, Knopman J. Middle meningeal artery embolization for recurrent chronic subdural hematoma: A case series. World Neurosurg. 2018. 118: e570-4
41. Mandai S, Sakurai M, Matsumoto Y. Middle meningeal artery embolization for refractory chronic subdural hematoma. Case report. J Neurosurg. 2000. 93: 686-8
42. Matsumoto R, Okada T, Mikuni N, Mitsueda-Ono T, Taki J, Sawamoto N. Hemispheric asymmetry of the arcuate fasciculus: A preliminary diffusion tensor tractography study in patients with unilateral language dominance defined by Wada test. J Neurol. 2008. 255: 1703-11
43. Mino M, Nishimura S, Hori E, Kohama M, Yonezawa S, Midorikawa H. Efficacy of middle meningeal artery embolization in the treatment of refractory chronic subdural hematoma. Surg Neurol Int. 2010. 1: 78
44. Moshayedi P, Liebeskind DS. Middle meningeal artery embolization in chronic subdural hematoma: Implications of pathophysiology in trial design. Front Neurol. 2020. 11: 923
45. Mulligan P, Raore B, Liu S, Olson JJ. Neurological and functional outcomes of subdural hematoma evacuation in patients over 70 years of age. J Neurosci Rural Pract. 2013. 4: 250-6
46. Mureb MC, Kondziolka D, Shapiro M, Raz E, Nossek E, Haynes J. DynaCT enhancement of subdural membranes after middle meningeal artery embolization: Insights into pathophysiology. World Neurosurg. 2020. 139: e265-70
47. Nakagawa I, Park HS, Kotsugi M, Wada T, Takeshima Y, Matsuda R. Enhanced hematoma membrane on DynaCT images during middle meningeal artery embolization for persistently recurrent chronic subdural hematoma. World Neurosurg. 2019. 126: e473-9
48. Nentwig MJ, Whitaker CM, Yang SY. Spinal subdural hygroma as a post-operative complication in revision spine fusion: A case report. J Surg Case Rep. 2019. 2019: rjz305
49. Ng HY, Ng WH, King NK. Value of routine early post-operative computed tomography in determining short-term functional outcome after drainage of chronic subdural hematoma: An evaluation of residual volume. Surg Neurol Int. 2014. 5: 136
50. Ng S, Derraz I, Boetto J, Dargazanli C, Poulen G, Gascou G. Middle meningeal artery embolization as an adjuvant treatment to surgery for symptomatic chronic subdural hematoma: A pilot study assessing hematoma volume resorption. J Neurointerv Surg. 2020. 12: 695-9
51. Nia AM, Srinivasan VM, Siddiq F, Thomas A, Burkhardt JK, Lall RR. Trends and outcomes of primary, rescue, and adjunct middle meningeal artery embolization for chronic subdural hematomas. World Neurosurg. 2022. 164: e568-73
52. Oh JW, Kim SH, Whang K. Traumatic cerebrospinal fluid leak: Diagnosis and management. Korean J Neurotrauma. 2017. 13: 63-7
53. Okuma Y, Hirotsune N, Sato Y, Tanabe T, Muraoka K, Nishino S. Midterm follow-up of patients with middle meningeal artery embolization in intractable chronic subdural hematoma. World Neurosurg. 2019. 126: e671-8
54. Ou Y, Fan W, Yu X, Wu L, Liu W. A single-center analysis of sex differences in patients with chronic subdural hematoma in China. Front Neurol. 2022. 13: 888526
55. Piergallini L, Dargazanli C, Derraz I, Costalat V. Immediate development of dural arteriovenous fistula after middle meningeal artery embolization: First angiographic demonstration. World Neurosurg. 2019. 128: 606-10.e1
56. Rovlias A, Theodoropoulos S, Papoutsakis D. Chronic subdural hematoma: Surgical management and outcome in 986 cases: A classification and regression tree approach. Surg Neurol Int. 2015. 6: 127
57. Sarma P, Garg M, Prem P, Gupta R. Embolization of the middle meningeal artery for the treatment of chronic subdural hematoma: A path less travelled so far. J Neurosci Rural Pract. 2022. 13: 471-5
58. Shah MM, Mandiga P, editors. Physiology, Plasma Osmolality and Oncotic Pressure. Treasure Island (FL): StatPearls Publishing; 2022. p.
59. Shotar E, Premat K, Barberis E, Talbi A, Lenck S, Cohen C. Dural arteriovenous fistula formation following bilateral middle meningeal artery embolization for the treatment of a chronic subdural hematoma: A case report. Acta Neurochir (Wien). 2021. 163: 1069-73
60. Shotar E, Meyblum L, Premat K, Lenck S, Degos V, Grand T. Middle meningeal artery embolization reduces the post-operative recurrence rate of at-risk chronic subdural hematoma. J Neurointerv Surg. 2020. 12: 1209-13
61. Sim YW, Min KS, Lee MS, Kim YG, Kim DH. Recent changes in risk factors of chronic subdural hematoma. J Korean Neurosurg Soc. 2012. 52: 234-9
62. Sirh S, Park HR, Park SQ. Usefulness of middle meningeal embolization to prevent recurrent spontaneous chronic subdural hemorrhage. J Cerebrovasc Endovasc Neurosurg. 2018. 20: 40-6
63. Soleman J, Nocera F, Mariani L. The conservative and pharmacological management of chronic subdural haematoma. Swiss Med Wkly. 2017. 147: w14398
64. Song DH, Kim YS, Chun HJ, Yi HJ, Bak KH, Ko Y. The predicting factors for recurrence of chronic subdural hematoma treated with burr hole and drainage. Korean J Neurotrauma. 2014. 10: 41-8
65. Stanišić M, Hald J, Rasmussen IA, Pripp AH, Ivanović J, Kolstad F. Volume and densities of chronic subdural haematoma obtained from CT imaging as predictors of postoperative recurrence: A prospective study of 107 operated patients. Acta Neurochir (Wien). 2013. 155: 323-33 discussion 333
66. Stubbs DJ, Vivian ME, Davies BM, Ercole A, Burnstein R, Joannides AJ. Incidence of chronic subdural haematoma: A single-centre exploration of the effects of an ageing population with a review of the literature. Acta Neurochir (Wien). 2021. 163: 2629-37
67. Tamez-Pérez HE, Quintanilla-Flores DL, RodríguezGutiérrez R, González-González JG, Tamez-Peña AL. Steroid hyperglycemia: Prevalence, early detection and therapeutic recommendations: A narrative review. World J Diabetes. 2015. 6: 1073-81
68. Tang J, Ai J, Macdonald RL. Developing a model of chronic subdural hematoma. Acta Neurochir Suppl. 2011. 111: 25-9
69. Tempaku A. A case of neuromyelitis optica diagnosed with a chronic subdural hematoma. J Rural Med. 2017. 12: 126-9
70. Tiwari A, Dmytriw AA, Bo R, Farkas N, Ye P, Gordon DS. Recurrence and coniglobus volumetric resolution of subacute and chronic subdural hematoma post-middle meningeal artery embolization. Diagnostics (Basel). 2021. 11: 257
71. Tommiska P, Raj R, Schwartz C, Kivisaari R, Luostarinen T, Satopää J. Finnish study of intraoperative irrigation versus drain alone after evacuation of chronic subdural haematoma (FINISH): A study protocol for a multicentre randomised controlled trial. BMJ Open. 2020. 10: e038275
72. Tripathy SR, Swarnakar PK, Mishra S, Mishra SS, Dhir MK, Behera SK. A review of sub acute subdural hematoma (SASDH) with our institutional experience and its management by double barrel technique (DbT): A novel technique. Surg Neurol Int. 2016. 7: S767-74
73. Wang H, Wang C, Li Z. Recurrent bilateral chronic subdural hematoma after interventional embolization combined with drilling and drainage treatment. J Craniofac Surg. 2020. 31: e171-3
74. Waqas M, Vakhari K, Weimer PV, Hashmi E, Davies JM, Siddiqui AH. Safety and effectiveness of embolization for chronic subdural hematoma: Systematic review and case series. World Neurosurg. 2019. 126: 228-36
75. Yadav YR, Parihar V, Namdev H, Bajaj J. Chronic subdural hematoma. Asian J Neurosurg. 2016. 11: 330-42
76. Yajima H, Kanaya H, Ogino M, Ueki K, Kim P. Middle meningeal artery embolization for chronic subdural hematoma with high risk of recurrence: A single institution experience. Clin Neurol Neurosurg. 2020. 197: 106097
77. Yokoya S, Nishii S, Takezawa H, Katsumori T, Takagi Y, Goto Y. Organized chronic subdural hematoma treated with middle meningeal artery embolization and small craniotomy: Two case reports. Asian J Neurosurg. 2020. 15: 421-4
78. Yu W, Chen W, Jiang Y, Ma M, Zhang W, Zhang X. Effectiveness comparisons of drug therapy on chronic subdural hematoma recurrence: A Bayesian network meta-analysis and systematic review. Front Pharmacol. 2022. 13: 845386
79. Zhu F, Wang H, Li W, Han S, Yuan J, Zhang C. Factors correlated with the postoperative recurrence of chronic subdural hematoma: An umbrella study of systematic reviews and meta-analyses. EClinicalMedicine. 2021. 43: 101234