- Unit of Neurosurgery, Azienda Socio SanitariaTerritoriale - Monza, Ospedale San Gerardo,
- Unit of Neuroradiology, Azienda Socio Sanitaria Territoriale - Monza, Ospedale San Gerardo, Monza,
- Unit of Neurosurgery, School of Medicine and Surgery, Department of Medicine and Surgery, Università degli Studi Milano Bicocca, Milan,
- Department of Biomedical, Metabolic and Neural Sciences, University of Modena and Reggio Emilia,
- Neurosurgery Division, University Hospital of Modena, Modena, Italy.
Correspondence Address:
Prof. Carlo Giorgio Giussani, Neurosurgery Unit, Azienda Socio Sanitaria Territoriale – Monza, Ospedale San Gerardo Via Pergolesi 33, 20900 Monza (MB) Italy. Department of Medicine and Surgery, Neurosurgery Unit, Università degli Studi Milano Bicocca School of Medicine, Milan, Italy.
DOI:10.25259/SNI_911_2021
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Andrea Di Cristofori1, Paolo Remida2, Mirko Patassini2, Lorenzo Piergallini2, Raffaella Buonanno1,3, Raffaele Bruno1,3, Giorgio Carrabba1,3, Giacomo Pavesi4,5, Corrado Iaccarino4,5, Carlo Giorgio Giussani1,3. Middle meningeal artery embolization for chronic subdural hematomas. A systematic review of the literature focused on indications, technical aspects, and future possible perspectives. 18-Mar-2022;13:94
How to cite this URL: Andrea Di Cristofori1, Paolo Remida2, Mirko Patassini2, Lorenzo Piergallini2, Raffaella Buonanno1,3, Raffaele Bruno1,3, Giorgio Carrabba1,3, Giacomo Pavesi4,5, Corrado Iaccarino4,5, Carlo Giorgio Giussani1,3. Middle meningeal artery embolization for chronic subdural hematomas. A systematic review of the literature focused on indications, technical aspects, and future possible perspectives. 18-Mar-2022;13:94. Available from: https://surgicalneurologyint.com/surgicalint-articles/11452/
Abstract
Background: Chronic subdural hematoma (CSDH) is one of the most common neurosurgical diseases that affect elderly and fragile patients and as a consequence, management can be challenging. Surgery represents the standard treatment; however, alternative options are under investigation. Middle meningeal artery (MMA) embolization is considered a minimally invasive treatment although with poor evidence. In this review, we tried to summarize the findings about MMA embolization as a treatment for a CSDH to provide a useful guidance for clinical practice and for future speculative aspects.
Methods: Literature review on PubMed until March 2021 was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement. We conducted a research on PubMed with a various combinations of the keywords “CSDH” and “middle meningeal artery” and “embolization,” “refractory subdural hematoma,” and then we reviewed the references of the relevant studies as additional source of eligible articles.
Results: Among the 35 studies eligible for this review, 22 were case series, 11 were case reports, one was a technical note, and 1 was a randomized trial. A total of 746 patients were found in the literature. Failure rate of MMA embolization was between 3.9 and 8.9% of the cases according the indication to treat CSDH (upfront vs. after surgery).
Conclusion: The global impression deriving from the data available and the literature is that MMA embolization is a safe procedure with very low complications and with a low failure rate, both when associated with surgery or in case of a standalone treatment.
Keywords: Chronic subdural hematoma, Middle meningeal artery embolization, Recurrent chronic subdural hematoma, Refractory subdural hematoma
INTRODUCTION
Chronic subdural hematoma (CSDH) is one of the most common neurosurgical diseases characterized by the presence of an abnormal fluid collection in the subdural space made of blood and blood degradation compounds.[
The overall incidence of CSDH ranges from 1.72 to 127.1/100.000 inhabitants, depending on the reports published in the literature, and it increases with age with the higher peak in patients over 65 years of age.[
For a subgroup of patients, a conservative approach may be proposed as first choice of treatment, but specific inclusion criteria for this “wait and see” management is far away to be clearly reported in literature.
Moreover, a conservative management, when indicated, is necessarily associated to a prolonged neuroradiological and clinical follow-up.[
In case of symptomatic CSDH, the standard treatment of care is mainly represented by surgical evacuation with several technical options described.[
The primary end-point of the surgical evacuation of CSDH is to release the intracranial hypertension and reduce the local mass effect.[
As a consequence, starting from the pathophysiology of CSDH, several pharmacological approaches have been proposed to avoid surgery, when possible, like use of corticosteroids, tranexamic acid, or ACE-inhibitors[
More recently, in addition to those strategies, an interventional approach under investigation is the devascularization of the external membrane of the CSDH through the embolization of the MMAs.[
The aim of our work is to provide an updated review about the findings regarding the efficacy of MMA embolization in CSDH with particular focus on open questions regarding this new technique:
When to propose MMA embolization as a standalone treatment. The efficacy of MMA embolization in preventing CSDH recurrences after surgical drainage. Possible criteria for selecting patients for MMA embolization.
MATERIALS AND METHODS
Literature review
We reviewed the existing literature on PubMed until March 22, 2021, in English language, without restrictions about the paper publication status, according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement.[
Afterward, all titles and abstracts were screened to exclude unrelated studies; this includes neurological conditions other than/causing other than CSDH (e.g., acute subdural hematomas, epidural hematomas, and tumor related CSDHs), studies about anatomy of MMA, studies without patients’ follow-up (either clinical or radiological) and spinal hematomas. Some other studies could have successively been excluded after full-text article reading.
Data of the eligible works were obtained through careful analysis of full text by one author and checked by another.
After having analyzed all the methods of the studies, patients were also classified according to indication for MMA embolization into the following:
Standalone embolization. Symptomatic CSDH (close to surgery to prevent recurrence or as prophylaxis for a recurrence). Recurrent CSDH. Recurrent after second surgery.
Case series with more than four patients and including a comparison with a control group were selected for further analyses and for comparison with our series of patients.
RESULTS
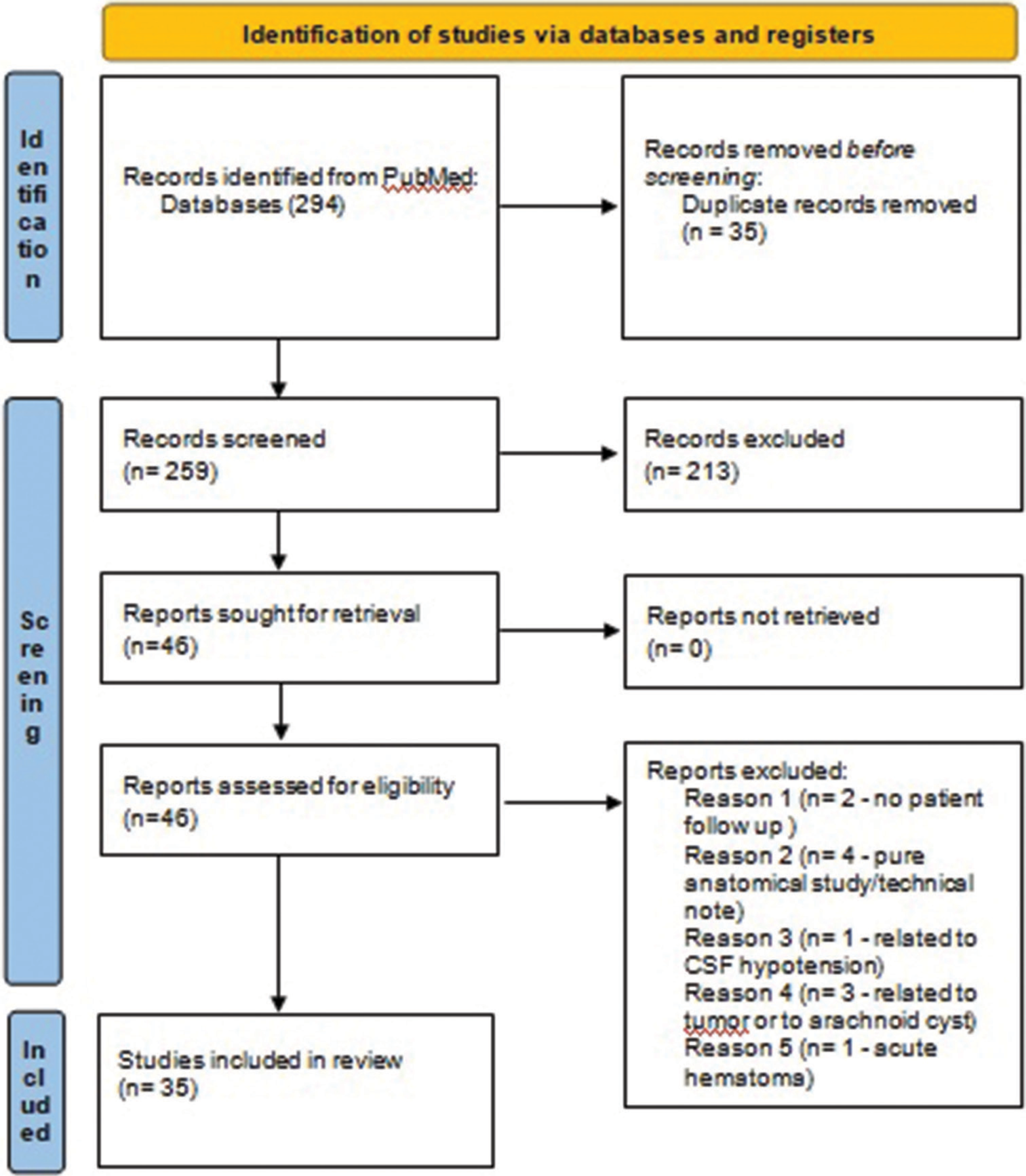
Throughout literature searching on PubMed (MEDLINE) 340 articles were reported with several combinations of the keywords described in the “methods” section. Four articles were eligible for reviewing after reference check. As a consequence, a total of 344 articles were screened for the review. Three-hundred and nine articles were excluded on the basis of the inclusion criteria of the present review [
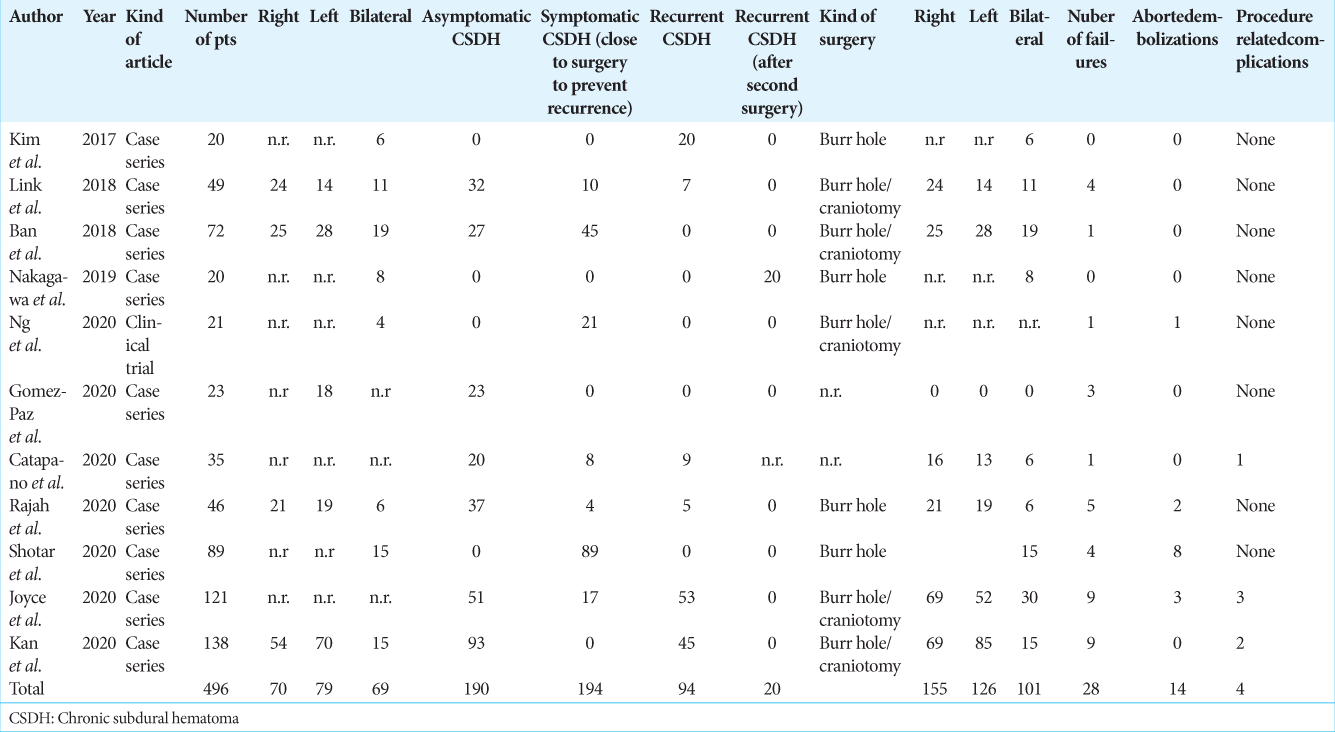
For each study, we extrapolated the indication for MMA embolization, the number of patients treated, the kind of study, the kind of surgery, the number of MMAs embolized, the number of treatment failures, the presence or absence of a control group, and the number of complications related to MMA embolization. All results are summarized in [
General considerations
Among the 35 studies eligible for this review, 22 were case series, 11 were case reports, one was a technical note, and one was a randomized trial. Among the 22 case series, five presented comparisons with an historical group of patients treated in a conventional way.
Starting from year 2000 with the first report by Mandai et al.[
A total of 746 patients have been described in literature till the date of the literature search. According to the published papers, we divided the studies reported in the literature into two groups; those that proposed MMA embolization only as a first treatment for a CSDH and those that proposed MMA embolization as an adjunctive treatment after surgical drainage. The last group included patients that underwent MMA embolization as prophylaxis for recurrence, patients that were treated with MMA embolization in case of CSDH recurrence and patients that underwent MMA embolization as a salvage option in case of multiple recurrences. This kind of subdivision is reported in [
MMA embolization alone for CSDH
MMA embolization was reported as a standalone treatment for a CSDH in 16/35 works and of these 16 studies, four were case reports. In these 16 works about standalone MMA embolization for CSDH treatment, a total of 309 patients (41.4% of total reported patients) were studied but some of them received a MMA embolization as a standalone treatment of a recurrent CSDH.
Indication for MMA embolization alone was different from one study to another [
Surgical rescue or refilling of subdural collection was considered the criteria for the failure of MMA embolization in all papers. In case MMA embolization was performed as a standalone treatment, the failure rate was 8.9% (8 patients out of 90); while the remaining patients experienced in all cases a resolution of the subdural collection at the follow-up CT scans. This failure rate was calculated taking into account only those studies in which it was possible to correlate treatment failure and indication to treat with MMA embolization alone. In some studies, like the one by Catapano et al., it is not specified who failed treatment with MMA embolization (patients treated for asymptomatic CSDHs with MMA embolization alone or patients treated for a recurrent CSDH after surgery?[
A comparison series of patients undergoing conventional treatment is reported only by Ban et al. in which no failure was associated with MMA embolization for these kinds of patients.[
MMA embolization after surgery for the prevention of CSDH recurrence or as a salvage treatment for recurrent CSDH
In 14/35 (40%) studies, MMA embolization was proposed as a prophylactic treatment to prevent recurrence and in 5 of them, indication for treatment overlapped between standalone treatment, prophylactic treatment and treatment at recurrence. Four works were case reports. These studies included a total of 305 patients (41.4% of total reported patients). One study did not report the number of patients treated in a prophylactic way making some of the results partly uncompleted.[
In general, patients were eligible for MMA embolization when they were considered high risk for CSDH recurrence but the way it was assessed differed among the studies. Okuma et al. in 2019 established a checklist for high risk CSDHs that were used to select patients at high risk and patients at low risk for recurrence.[
In 14/35 studies MMA embolization was proposed as an alternative treatment after surgery for recurrent CSDH to prevent a second surgery. Patients included in this group were 158 accounting for 21.2% of the total cases described in literature. Three of the studies in this group were case reports.
In 8/34 studies, MMA embolization was proposed as a salvage treatment after second recurrence. The majority of the publications in this group were produced between the years 2000 and 2015; year after which the number of patients included in case series increased. In this scenario MMA embolization was performed on 35 patients (4.6% of the total number of patients) of which 18/35 were described between the year 2000 and the year 2015.
Failure rates of MMA embolization and procedural complications
Analyzing the 746 patients reported in the literature, 41 patients reported a failure of MMA embolization to prevent surgery or hematoma refilling with an overall failure rate of 5.5% while considering the series with more than 20 patients included, the failure rate remained quite the same at 5.6% with 28 patients on a total of 496 reported in 11 works [
As reported above, failure rate after MMA embolization alone was 8.9% while in case of MMA performed as a recurrence prophylaxis after surgery, the failure rate was 3.9% (six patients out of 195). In case of MMA embolization performed after CSDH recurrence it was not possible to measure the failure rate since only the study by Link in 2018 was described a failure on a series of 6 patients;[
Complications of MMA embolization was reported in six cases with a consequent very low rate with a global rate of 0.8% on 746 patients. Treatment failure was considered as a separate complication. Complications encompassed one cerebral infarction, one seizure, one intermittent aphasia, one cerebrovascular complication, one cerebrovascular infarction, and one acute worsening of CSDH.
Studies including a comparison group with conventional treatment
In the literature, five studies encompassed a comparison group. In five studies a comparison group made by an historical cohort of patients treated with conventional technique.
In total, the control groups of the five studies included 727 patients whose outcome was compared with 205 patients undergoing MMA embolization with heterogeneous indications.
Globally, patients that received MMA embolization showed a lower recurrence rate and required less surgical procedures. In fact, the failure rate of MMA embolization in preventing surgery was 3.9% (6 patients on 155) while the recurrence rate after conventional treatment in historical cohorts was 29.5% (214 patients on 727 patients). Most of the patients were treated with MMA embolization for recurrent hematomas while only 27 patients enrolled in the work by Ban et al. were treated with MMA embolization alone with no cases of failure.[
DISCUSSION
Considerations about indications for MMA embolization
From the analysis of the literature, we identified four indications for MMA embolization:
To prevent surgery in patients with paucisymptomatic CSDHs,[ As prophylaxis of a recurrence after a first surgery,[ After a first recurrence to prevent further recurrence,[ As an adjunctive treatment in case of a second surgery.[
The first scenario is completely different; after standalone MMA embolization the remaining subdural fluid collection may be at risk of progression due to its pro-inflammatory features. In the other scenarios with the combined treatment, surgical evacuation can decrease the pro-inflammatory and vasogenic content of the subdural fluid collection, while the vascularization of the parietal membrane of the CSDH itself is reduced by MMA embolization.[
Thus, this pathophysiological difference may be related to the lower failure rate observed in CSDHs treated with combined procedures respect to the first scenario (3.9% vs. about 8.9%).
Standalone MMA embolization has been successfully achieved in all 27 cases reported by Ban et al. in 2017;[
Finally, the high rate of successful MMA embolization after first surgical evacuation or after recurrence should take in consideration a fraction of patients who could still have recovered completely after surgery even without MMA embolization and the low risk of further recurrences.
In fact, among a series of 372 patients treated for a CSDH, only 20 cases (5.4%) experienced a third recurrence that underwent MMA embolization as reported by Waqas et al. in 2017.[
This is summarized in [
At present, MMA embolization represents the first therapeutic option in the majority of cases reported in literature.[
Number of MMA embolization, number of patients treated and outcome
The total amount of the number of patients that underwent MMA embolization is not well reported in literature. Some authors provide the number of CSDH treated but not the number of patients, such as in the series reported by Santiago Gomez-Paz et al. or by Joyce et al.,[
In addition, the indication to proceed with the MMA embolization is not always well reported. Yajima et al. included patients with an objectively inclusion criteria as a third recurrence, and a more subjective inclusion criteria as the risk of recurrence without differentiating the results for each of these two groups.[
Moreover, in case of patients included for a standalone MMA embolization, there is no agreement about eligibility of candidates. While a poor symptom set seems to be generally reported as clinical inclusion criteria, conversely some neuroradiological data, like the midline shift, are considered exclusion criteria by Ban et al.[
Should we consider a standardized way for describing case series in view of future speculations?
The difficulty in systematically reviewing the data of the literature is the heterogeneity of data itself. The number of patients treated should be considered differently from the number of CSDHs treated and, in particular, bilateral CSDHs should be considered a category of patients separately due to their predisposition to develop a recurrent hematoma.
Patients should also be categorized by indication for MMA embolization given the different rate of procedure failure with regards to the indications for it and additionally the time between surgery and MMA embolization should be specified. This would allow to understand if there is a maximal length of time to perform a MMA embolization.
Moreover, patients under antiplatelet or anticoagulant medications could be considered for separate studies since they sometimes represent a clinical challenge (patients at risk for acute hemorrhages after surgery or at risk for complications related with drug discontinuation). In fact, contrary to what happens for surgical drainage, MMA embolization can be performed without suspending administration of prohemorrhagic drugs. Moreover, MMA embolization in addition to surgery may be of help in reducing the risk of CSDH recurrence and consequently, the complications related to discontinuation of anti-platelet or anticoagulant medications.
Although the great heterogeneity of the cases found in the literature, past experiences are of great importance in leading the neurosurgical community to trace a way to understand the efficacy and usefulness of invasive treatments. According to the experiences reported in literature, two points are of paramount importance in depicting future scenarios and they are related to the presence of the two main groups of patients with CSDH treated with MMA embolization; one group treated with standalone MMA embolization and the other treated after surgery.
First, from a pathophysiological point of view, the clear cut between these two groups is due to the presence of a residual CSDH collection that can reduce the efficacy of MMA embolization. Indirect evidence is related with the lower failure rate in case MMA embolization is performed soon after surgery. In this view, the neurosurgical community might look forward to better understand if some patients affected by a CSDH may have the opportunity to avoid a surgical drainage of the CSDH. A clear demonstration of such evidence would have a significant impact on social and economic costs since MMA embolization alone can be managed as a day-case procedure as proposed during the SARS-Cov2 pandemic.[
Second, performing MMA embolization after surgery may lead to reduction of CSDH recurrence rate and may reduce the timing needed for CSDH resolution leading to a possible consequent reduction in in-hospital days, morbidity and mortality due to hospitalization. Finally, reduction in the number of surgeries required in case of recurrence may reduce the economic costs of the CSDH disease. Taken together, these considerations would lead to a global benefit to the community since CSDH incidence is expected to increase over the next years.[
Starting from these considerations, two kind of randomized clinical trials would be needed: one comparing conventional surgery versus standalone MMA embolization for poorly symptomatic subdural collections and one comparing conventional surgery versus surgery plus MMA embolization for CSDHs. In July 2020, Ng et al. published the first clinical trial on CSDH treatment with MMA embolization. They performed a randomized clinical trial allocating 21 patients for surgery alone and 25 patients for surgery + MMA embolization. They published a preliminary report of their trial demonstrating that MMA embolization after surgery reduces the time needed for CSDH absorption.[
CONCLUSION
In this review, we tried to summarize the findings about MMA embolization as a treatment for a CSDH in order to provide a useful guidance for the clinical practice and for future speculative aspects about alternative CSDH treatments. A significant limitation of our work is related with the lack of distinction in several works about the failure rates of MMA embolization according to the indication for which the treatment has been proposed. As a consequence, it is not possible to understand which patient failed to respond to MMA embolization (upfront treatment vs. postsurgical) and it is not possible to draw the exact number of patients but only the exact number of subdural collections that may affect the same patient or different patients. However, the global impression deriving from the data available and the literature is that in the near future, MMA embolization is a safe procedure with very low complications and with a low failure rate and will probably become one of the standards of care.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Abboud T, Dührsen L, Gibbert C, Westphal M, Martens T. Influence of antithrombotic agents on recurrence rate and clinical outcome in patients operated for chronic subdural hematoma. Neurocirugía. 2018. 29: 86-92
2. Alford EN, Rotman LE, Erwood MS, Oster RA, Davis MC, Pittman HB. Development of the subdural hematoma in the elderly (SHE) score to predict mortality. J Neurosurg ry. 2019. 132: 1616-22
3. Andersen-Ranberg NC, Debrabant B, Poulsen FR, Bergholt B, Hundsholt T, Fugleholm K. The Danish chronic subdural hematoma study predicting recurrence of chronic subdural hematoma. Acta Neurochir. 2019. 161: 885-94
4. Andersen-Ranberg NC, Poulsen FR, Bergholt B, Hundsholt T, Fugleholm K. Bilateral chronic subdural hematoma: Unilateral or bilateral drainage?. J Neurosurg. 2016. 126: 1905-11
5. Arham A, Zaragita N. Middle meningeal artery embolization following burr hole in chronic subdural hematoma. Asian J Neurosurg. 2020. 15: 382-4
6. Asghar M, Adhiyaman V, Greenway MW, Bhowmick BK, Bates A. Chronic subdural haematoma in the elderly--a North Wales experience. J R Soc Med. 2002. 95: 290-2
7. Ban SP, Hwang G, Byoun HS, Kim T, Lee SU, Bang JS. Middle meningeal artery embolization for chronic subdural hematoma. Radiology. 2018. 286: 992-9
8. Bucher B, Maldaner N, Regli L, Sarnthein J, Serra C. Standardized assessment of outcome and complications in chronic subdural hematoma: Results from a large case series. Acta Neurochir. 2019. 161: 1297-304
9. Catapano JS, Ducruet AF, Nguyen CL, Baranoski JF, Cole TS, Majmundar N. Middle meningeal artery embolization for chronic subdural hematoma: An institutional technical analysis. J Neurointerv Surg. 2020. 13: 657-60
10. Chihara H, Imamura H, Ogura T, Adachi H, Imai Y, Sakai N. Recurrence of a refractory chronic subdural hematoma after middle meningeal artery embolization that required craniotomy. NMC Case Rep J. 2014. 1: 1-5
11. Dumont TM, Rughani AI, Goeckes T, Tranmer BI. Chronic subdural hematoma: A sentinel health event. World Neurosurg. 2013. 80: 889-92
12. Edlmann E, Giorgi-Coll S, Whitfield PC, Carpenter KL, Hutchinson PJ. Pathophysiology of chronic subdural haematoma: Inflammation, angiogenesis and implications for pharmacotherapy. J Neuroinflammation. 2017. 14: 108
13. Entezami P, Field NC, Dalfino JC. Outpatient management of chronic expanding subdural hematomas with endovascular embolization to minimize inpatient admissions during the COVID-19 viral pandemic. Interv Neuroradiol. 2021. 27: 716-21
14. Entezami P, Nourollahzadeh E, Dalfino J. Embolization of middle meningeal artery for the treatment of headaches induced by chronic subdural hematoma: A case report. Headache. 2019. 59: 615-8
15. Gomez-Paz S, Akamatsu Y, Salem MM, EnriquezMarulanda A, Robinson TM, Ogilvy CS. Upfront middle meningeal artery embolization for treatment of chronic subdural hematomas in patients with or without midline shift. Interv Neuroradiol. 2021. 27: 571-6
16. Guidry BS, Kelly KA, Yengo-Kahn AM, Lan M, Tang AR, Chotai S. Statins as a medical adjunct in the surgical management of chronic subdural hematomas. World Neurosurg. 2021. 149: e281-91
17. Hashimoto T, Ohashi T, Watanabe D, Koyama S, Namatame H, Izawa H. Usefulness of embolization of the middle meningeal artery for refractory chronic subdural hematomas. Surg Neurol Int. 2013. 4: 104
18. Hirai S, Ono J, Odaki M, Serizawa T, Nagano O. Embolization of the middle meningeal artery for refractory chronic subdural haematoma: Usefulness for patients under anticoagulant therapy. Interv Neuroradiol. 2004. 10: 101-4
19. Joyce E, Bounajem MT, Scoville J, Thomas AJ, Ogilvy CS, Riina HA. Middle meningeal artery embolization treatment of nonacute subdural hematomas in the elderly: A multiinstitutional experience of 151 cases. Neurosurg Focus. 2020. 49: E5
20. Kan P, Maragkos GA, Srivatsan A, Srinivasan V, Johnson J, Burkhardt JK. Middle meningeal artery embolization for chronic subdural hematoma: A multi-center experience of 154 consecutive embolizations. Neurosurgery. 2021. 88: 268-77
21. Kim E. Embolization therapy for refractory hemorrhage in patients with chronic subdural hematomas. World Neurosurg. 2017. 101: 520-7
22. Lee KS. Review natural history of chronic subdural haematoma. Brain Inj. 2004. 18: 351-8
23. Link TW, Boddu S, Paine SM, Kamel H, Knopman J. Middle meningeal artery embolization for chronic subdural hematoma: A series of 60 cases. Neurosurgery. 2019. 85: 801-7
24. Link TW, Schwarz JT, Paine SM, Kamel H, Knopman J. Middle meningeal artery embolization for recurrent chronic subdural hematoma: A case series. World Neurosurg. 2018. 118: e570-4
25. Mandai S, Sakurai M, Matsumoto Y. Middle meningeal artery embolization for refractory chronic subdural hematoma. Case report. J Neurosurg. 2000. 93: 686-8
26. Matsumoto R, Okada T, Mikuni N, Mitsueda-Ono T, Taki J, Sawamoto N. Hemispheric asymmetry of the arcuate fasciculus: a preliminary diffusion tensor tractography study in patients with unilateral language dominance defined by Wada test. J Neurol. 2008. 255: 1703-11
27. Miah IP, Herklots M, Roks G, Peul WC, Walchenbach R, Dammers R. Dexamethasone therapy in symptomatic chronic subdural hematoma (DECSA-R): A retrospective evaluation of initial corticosteroid therapy versus primary surgery. J Neurotrauma. 2020. 37: 366-72
28. Mino M, Nishimura S, Hori E, Kohama M, Yonezawa S, Midorikawa H. Efficacy of middle meningeal artery embolization in the treatment of refractory chronic subdural hematoma. Surg Neurol Int. 2010. 1: 78
29. Miranda LB, Braxton E, Hobbs J, Quigley MR. Chronic subdural hematoma in the elderly: Not a benign disease. J Neurosurg. 2011. 114: 72-6
30. Moffatt CE, Hennessy MJ, Marshman LA, Manickam A. Long-term health outcomes in survivors after chronic subdural haematoma. J Clin Neurosci. 2019. 66: 133-7
31. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009. 6: e1000097
32. Mureb MC, Kondziolka D, Shapiro M, Raz E, Nossek E, Haynes J. DynaCT Enhancement of subdural membranes after middle meningeal artery embolization: Insights into pathophysiology. World Neurosurg. 2020. 139: e265-70
33. Nakagawa I, Park HS, Kotsugi M, Wada T, Takeshima Y, Matsuda R. Enhanced hematoma membrane on dynact images during middle meningeal artery embolization for persistently recurrent chronic subdural hematoma. World Neurosurg. 2019. 126: e473-9
34. Ng S, Derraz I, Boetto J, Dargazanli C, Poulen G, Gascou G. Middle meningeal artery embolization as an adjuvant treatment to surgery for symptomatic chronic subdural hematoma: A pilot study assessing hematoma volume resorption. J Neurointerv Surg. 2020. 12: 695-9
35. Okuma Y, Hirotsune N, Sato Y, Tanabe T, Muraoka K, Nishino S. Midterm follow-up of patients with middle meningeal artery embolization in intractable chronic subdural hematoma. World Neurosurg. 2019. 126: e671-8
36. Piergallini L, Dargazanli C, Derraz I, Costalat V. Immediate development of dural arteriovenous fistula after middle meningeal artery embolization: First angiographic demonstration. World Neurosurg. 2019. 128: 606-10.e1
37. Rajah GB, Waqas M, Dossani RH, Vakharia K, Gong AD, Rho K. Transradial middle meningeal artery embolization for chronic subdural hematoma using Onyx: Case series. J NeuroInterv Surg. 2020. 12: 1214-8
38. Rauhala M, Helén P, Huhtala H, Heikkilä P, Iverson GL, Niskakangas T. Chronic subdural hematoma incidence, complications, and financial impact. Acta Neurochir. 2020. 162: 2033-43
39. Scerrati A, Visani J, Ricciardi L, Dones F, Rustemi O, Cavallo MA. To drill or not to drill, that is the question: Nonsurgical treatment of chronic subdural hematoma in the elderly. A systematic review. Neurosurg Focus. 2020. 49: E7
40. Shapey J, Glancz LJ, Brennan PM. Chronic subdural haematoma in the elderly: Is it time for a new paradigm in management?. Curr Geri Rep. 2016. 5: 71-7
41. Shotar E, Meyblum L, Premat K, Lenck S, Degos V, Grand T. Middle meningeal artery embolization reduces the post-operative recurrence rate of at-risk chronic subdural hematoma. J Neurointerv Surg. 2020. 12: 1209-13
42. Shotar E, Premat K, Barberis E, Talbi A, Lenck S, Cohen C. Dural arteriovenous fistula formation following bilateral middle meningeal artery embolization for the treatment of a chronic subdural hematoma: A case report. Acta Neurochir. 2021. 163: 1069-73
43. Sirh S, Park HR, Park SQ. Usefulness of middle meningeal embolization to prevent recurrent spontaneous chronic subdural hemorrhage. J Cerebrovasc Endovasc Neurosurg. 2018. 20: 40
44. Srivatsan A, Mohanty A, Nascimento FA, Hafeez MU, Srinivasan VM, Thomas A. Middle meningeal artery embolization for chronic subdural hematoma: Meta-analysis and systematic review. World Neurosurg. 2019. 122: 613-9
45. Sun H, Zhao JZ. Application of intraoperative ultrasound in neurological surgery. Minim Invasive Neurosurg. 2007. 50: 155-9
46. Tanaka T, Kaimori M. Histological study of vascular structure between the dura mater and the outer membrane in chronic subdural hematoma in an adult. No Shinkei Geka. 1999. 27: 431-6
47. Tempaku A. A case of neuromyelitis optica diagnosed with a chronic subdural hematoma. J Rural Med. 2017. 12: 126-9
48. Tiwari A, Dmytriw AA, Bo R, Farkas N, Ye P, Gordon DS. Recurrence and coniglobus volumetric resolution of subacute and chronic subdural hematoma post-middle meningeal artery embolization. Diagnostics. 2021. 11: 257
49. Uno M, Toi H, Hirai S. Chronic subdural hematoma in elderly patients: Is this disease benign?. Neurol Med Chir (Tokyo). 2017. 57: 402-9
50. Wang H, Wang C, Li Z. Recurrent bilateral chronic subdural hematoma after interventional embolization combined with drilling and drainage treatment. J Craniofac Surg. 2020. 31: e171-3
51. Waqas M, Vakhari K, Weimer PV, Hashmi E, Davies JM, Siddiqui AH. Safety and effectiveness of embolization for chronic subdural hematoma: Systematic review and case series. World Neurosurg. 2019. 126: 228-36
52. Yajima H, Kanaya H, Ogino M, Ueki K, Kim P. Middle meningeal artery embolization for chronic subdural hematoma with high risk of recurrence: A single institution experience. Clin Neurol Neurosurg. 2020. 197: 106097
53. Yang W, Huang J. Chronic subdural hematoma. Neurosurg Clin N Am. 2017. 28: 205-10
54. Yokoya S, Nishii S, Takezawa H, Katsumori T, Takagi Y, Goto Y. Organized chronic subdural hematoma treated with middle meningeal artery embolization and small craniotomy: Two case reports. Asian J Neurosurg. 2020. 15: 421
55. Yun H, Ding Y. How to remove those bloody collections: Nonsurgical treatment options for chronic subdural hematoma. Brain Circ. 2020. 6: 254