- Division of Neurological Surgery, University of Hawaii John A. Burns School of Medicine, Honolulu, Hawaii, United States.
Correspondence Address:
Frances Tiffany Cava Morden, Division of Neurological Surgery, University of Hawaii John A. Burns School of Medicine, Honolulu, Hawaii, United States.
DOI:10.25259/SNI_1051_2022
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Frances Tiffany Cava Morden, Clark Gianni Caballero, Maveric Abella, Andie Conching, Hannah Gang, Thomas Noh. Middle meningeal artery embolization for symptomatic chronic subdural hematoma in the setting of severe transfusion-refractory thrombocytopenia: A case study and review of literature. 30-Jun-2023;14:223
How to cite this URL: Frances Tiffany Cava Morden, Clark Gianni Caballero, Maveric Abella, Andie Conching, Hannah Gang, Thomas Noh. Middle meningeal artery embolization for symptomatic chronic subdural hematoma in the setting of severe transfusion-refractory thrombocytopenia: A case study and review of literature. 30-Jun-2023;14:223. Available from: https://surgicalneurologyint.com/surgicalint-articles/12388/
Abstract
Background: Surgical decompression for the treatment of chronic subdural hematomas (cSDHs) is irrefutably effective; however, its utility in managing cSDH in patients with comorbid coagulopathy remains controversial. The optimal threshold for platelet transfusion in cSDH management is 3, according to guidelines from the American Association of Blood Banks GRADE framework. This threshold may be unachievable in refractory thrombocytopenia, though surgical intervention may still be warranted. We present a patient with symptomatic cSDH and transfusion-refractory thrombocytopenia successfully treated with middle meningeal artery embolization (eMMA). We also review the literature to identify management approaches for cSDH with severe thrombocytopenia.
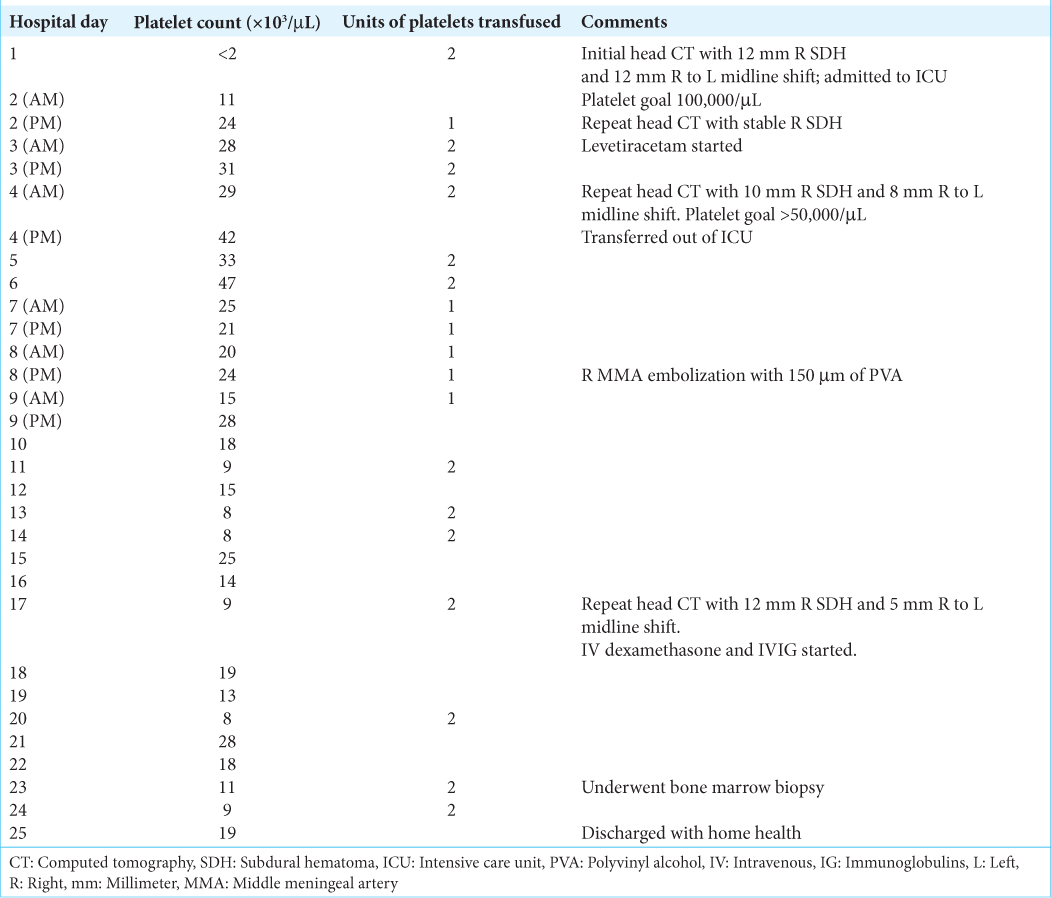
Case Description: A 74-year-old male with acute myeloid leukemia presented to the emergency department with persistent headache and emesis following fall without head trauma. Computed tomography (CT) revealed a 12 mm right-sided, mixed density SDH. Platelets were 3 initially, which stabilized to 20,000 following platelet transfusions. He then underwent right eMMA without surgical evacuation. He received intermittent platelet transfusions with platelet goal >20,000 and was discharged on hospital day 24 with resolving SDH on CT.
Conclusion: High-risk surgical patients with refractory thrombocytopenia and symptomatic cSDH may be successfully treated with eMMA without surgical evacuation. A platelet goal of 20,000/mm3 before and following surgical intervention proved beneficial for our patient. Similarly, a literature review of seven cases of cSDH with comorbid thrombocytopenia revealed five patients undergoing surgical evacuation following initial medical management. Three cases reported a platelet goal of 20,000. All seven cases resulted in stable or resolving SDH with platelets >20,000 at discharge.
Keywords: Chronic subdural hematoma, Middle meningeal artery embolization, Platelet goal, Platelet transfusion, Thrombocytopenia
INTRODUCTION
Chronic subdural hematoma (cSDH) is a common complication of acute myeloid leukemia (AML).[
Treatments for acute myeloid leukemia (AML) like chemotherapy and hypomethylating agents put patients at increased risk for thrombocytopenia and intracranial hemorrhage,[
Middle meningeal artery embolization (eMMA) for cSDH is an emerging treatment modality.[
To the best of our knowledge, there has been no report of a patient with AML, symptomatic cSDH, and transfusion-refractory thrombocytopenia treated with eMMA. We present a case of improving chronic SDH in the setting of AML-related thrombocytopenia by eMMA without surgical evacuation. We also performed a systematic review of the literature to identify management strategies for cSDH with severe thrombocytopenia.
CASE PRESENTATION
A 74-year-old man with a history of AML presented to the emergency department (ED) for a complaint of nonresolving throbbing headache with nausea and vomiting after a witnessed fall without head trauma. He was on his second cycle of therapy with venetoclax and a hypomethylating agent.
At presentation to the ED, he had left upper extremity and left lower extremity drift with 4/5 left upper and lower extremity weakness, and scattered ecchymoses with a petechial rash. Laboratory studies were significant for severe thrombocytopenia of <2000/µL in the ED. Computed tomography of the head (hCT) revealed a right sided, mixed density 12 mm SDH with a 12 mm leftward midline shift and patchy acute subarachnoid hemorrhages [
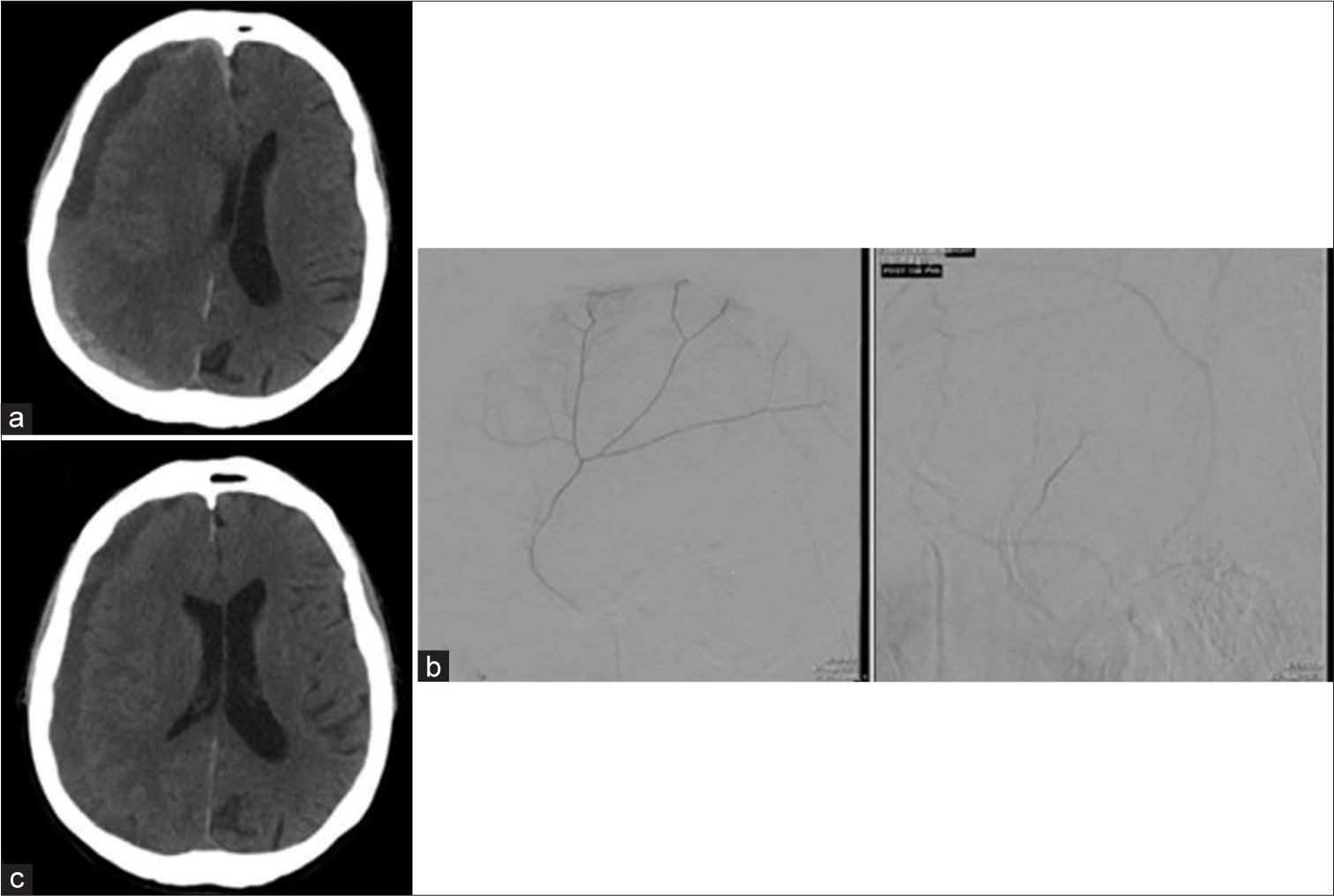
Figure 1:
Evolution of subdural hematoma (SDH) from presentation to 45 days later via head computed tomography (CT). (a) Head CT at initial presentation revealed a right-sided, mixed density 12 mm SDH with 12 mm midline shift to the left. (b) Cerebral angiogram visualizing middle meningeal artery. (c) Repeat head CT on hospital day 17 showing stable right SDH with resolving midline shift (5 mm to left).
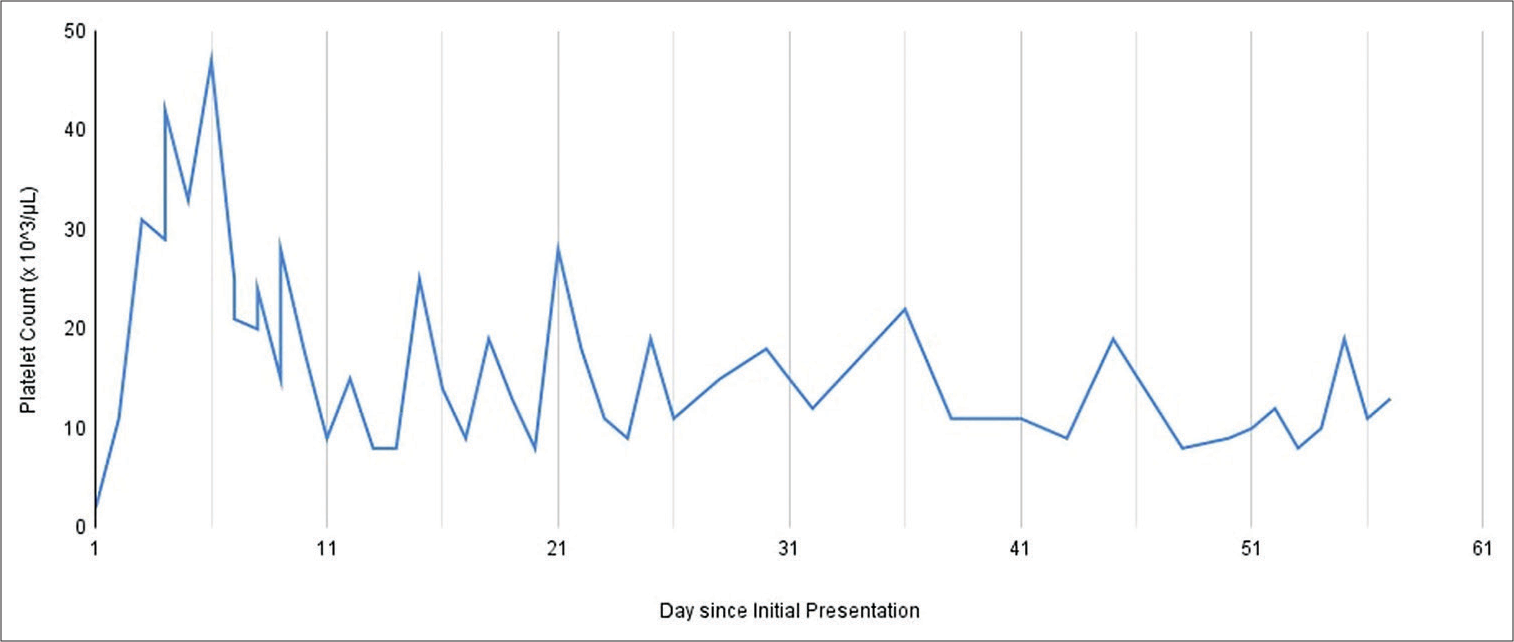
Figure 2:
Graphical depiction of patient’s platelet counts following initial presentation. The patient’s platelet count ranged from <2000/mm3 at initial presentation up to 48,000 5 days after presentation following multiple platelet transfusions. He underwent eMMA 7 days after presentation with platelet count of 20,000. He was discharged on 23 days after presentation and received outpatient platelet transfusions 3 times a week with a platelet goal of 20,000. His average platelet count was 14,000.
Fifty days after his initial presentation, the patient was readmitted for acute kidney injury secondary to severe tumor lysis syndrome, acute colitis, acute left pyelonephritis, anemia with hemoglobin of 7.8 g/dL, and thrombocytopenia with platelet count of 9000/µL. On hospital day 2, hCT showed a grossly stable SDH. He was considered a poor candidate for hemodialysis due to AML, discharged to hospice on hospital day 7, and passed 4 days later.
Systematic review
The optimal management of cSDHs in patients with concurrent thrombocytopenia remains obscure. In this review, we sought to find case reports or series similar to our patient to identify management strategies and treatment efficacy for cSDH with severe thrombocytopenia currently documented in the literature.
The preferred reporting items for systematic reviews and meta-analyses (PRISMA) reporting guideline were implemented for this review. A systematic search of PubMed and Web of Science data was conducted on October 13, 2021. The following search terms were utilized: thrombocytopenia OR pancytopenia; subdural; hematoma OR hemorrhage OR bleed*. Inclusion criteria required detection of isodense or hypodense areas in the subdural space on CT and report of individual patient data on platelet counts at intracranial cSDH confirmation, management of cSDH, platelet count following management, and outcome. Patients <18 years old and patients taking anticoagulants before cSDH presentation were excluded from the study. Reviews, editorials, and non-English articles were also excluded from the study. Two independent authors assessed the articles for inclusion and exclusion criteria.
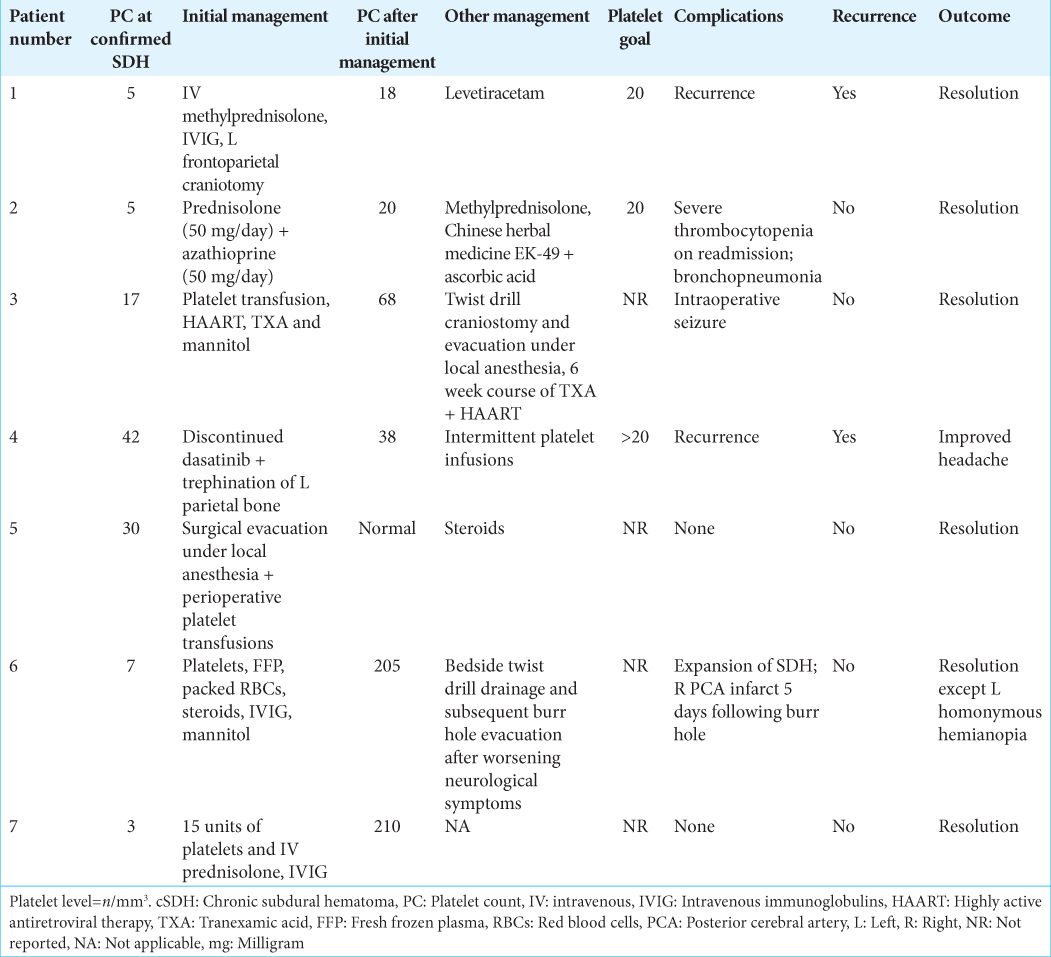
Individual patient data on the following were collected: age, sex, risk factors/etiology of thrombocytopenia, location of SDH, size of SDH, presence of midline shift, platelet counts at the time of SDH confirmation and after management, management of SDH and thrombocytopenia, platelet goal for management, complications, recurrence of SDH, and outcome at last follow-up. These data were collected by one author and reviewed by another author.
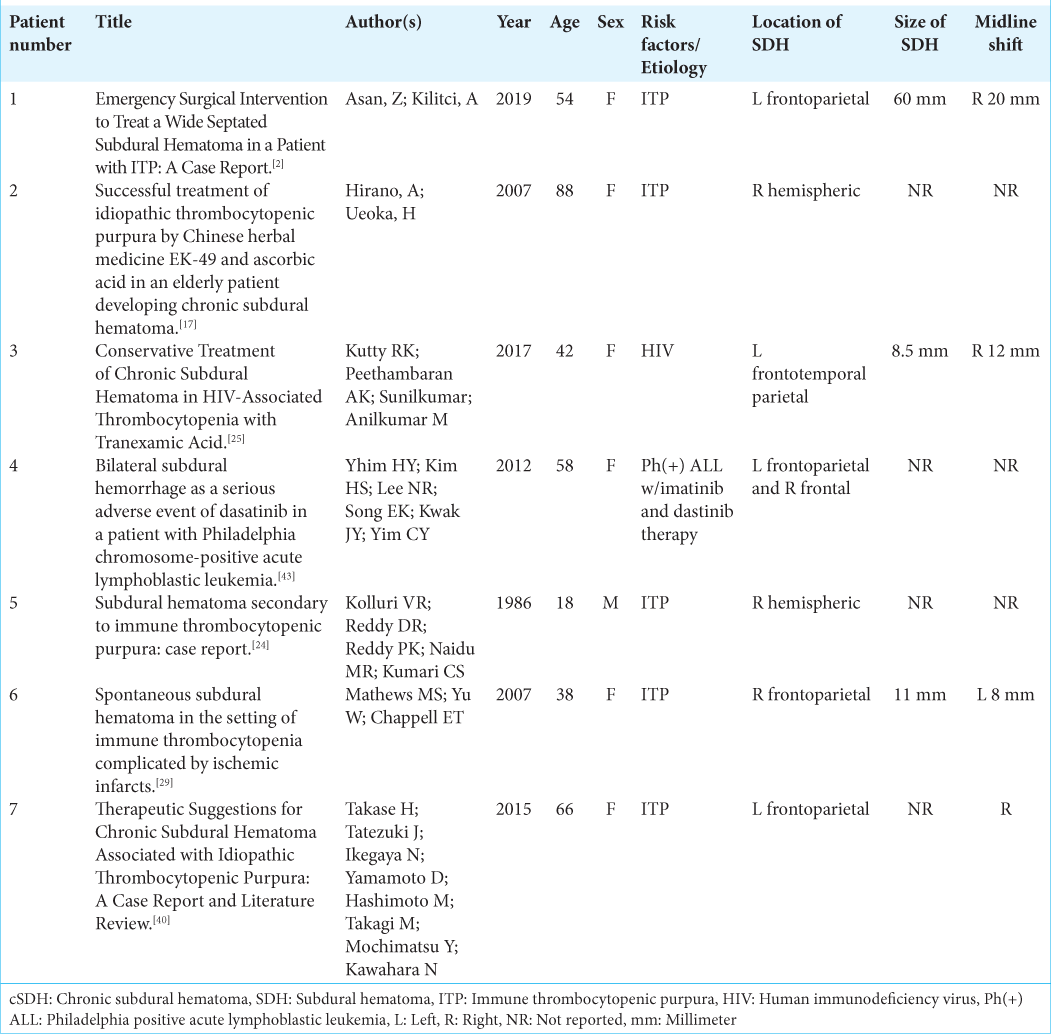
Seven out of 242 studies met inclusion criteria.[
Figure 4:
Preferred reporting items for systematic reviews and meta-analyses flow chart of systematic review conducted in October 2021. A total of 242 unique records were identified using PubMed and Web of Science databases. Initial title and abstract screening excluded 97 articles. Full-text screening found seven articles meeting inclusion criteria, n: Number.
DISCUSSION
Previous studies on safe platelet ranges for surgical management of SDHs focused on platelet transfusions for achieving platelet goals.[
The medical and surgical management varied widely among cases. Complications varied with two of the seven cases (Patient 1 and 4) having recurrence of the SDH after treatment. Patient 1 was diagnosed with concurrent ITP with the left-sided SDH and underwent left frontoparietal craniotomy with IV methylprednisolone and IVIG. Patient 4 had philadelphia chromosome positive ALL with bilateral SDH while receiving imatinib and dasatinib therapy and underwent trephination. Thrombocytopenia induced by hematologic disorders, such as ITP and ALL predisposed these patients to higher risk of recurrence after initial surgical management for SDH. Circulating autoantibodies due to the ITP present in Patient 1 led to a diminished recovery rate in endogenous platelet counts. ALL is known to lead to marrow failure contributing to decreased platelet count, making it difficult to recover adequate platelet numbers to prevent recurrent bleeding. This is the first report of eMMA for stabilization of cSDH without recurrence in the setting of refractory thrombocytopenia.
Once thought to be due to a singular event, the central pathology of SDHs is now being recognized as a cerebrovascular event with a cycle of hemorrhage, fibrosis, and angiogenesis.[
The MMA is believed to supply the outer membrane of a SDH,[
Although the exact mechanism of eMMA remains unclear, our findings support its use in populations with persistent refractory thrombocytopenia to prevent further bleeding. In this case, the patient’s cSDH was stable enough to be managed outside the hospital, so he spent his last moments with his loved ones as per his advanced directive. The overall treatment benefit among patients with substantial disease burden should also be emphasized in the management of cSDH.
CONCLUSION
Our case has shown the success of eMMA without surgical evacuation in successfully treating symptomatic cSDH in a high-risk surgical patient with refractory thrombocytopenia. Based on this case and a systematic review of seven cases of cSDH with comorbid thrombocytopenia, a platelet goal of 20,000/mm3 prior to any surgical intervention may be sufficient and more attainable than current platelet goals of 40,000-50,000/mm3. Though all patients included in this review had stable or resolving cSDH, further investigations are required to determine the utility of lowering the platelet threshold prior to neurosurgical intervention.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgments
The authors would like to acknowledge Andrea Siu for facilitating the logical aspects of this study. Thank you to the medical institution for assistance with data retrieval.
References
1. Abdelfatah M. Management of chronic subdural hematoma in patients with intractable thrombocytopenia. Turk Neurosurg. 2016. 28: 400-4
2. Asan Z, Kilitci A. Emergency surgical intervention to treat a wide septated subdural hematoma in a patient with ITP: A case report. Cyprus J Med Sci. 2019. 4: 63-5
3. Balser D, Farooq S, Mehmood T, Reyes M, Samadani U. Actual and projected incidence rates for chronic subdural hematomas in United States Veterans Administration and civilian populations. J Neurosurg. 2015. 123: 1209-15
4. Ban SP, Hwang G, Byoun HS, Kim T, Lee SU, Bang JS. Middle meningeal artery embolization for chronic subdural hematoma. Radiology. 2018. 286: 992-9
5. Bounajem MT, Campbell RA, Denorme F, Grandhi R. Paradigms in chronic subdural hematoma pathophysiology: Current treatments and new directions. J Trauma Acute Care Surg. 2021. 91: e134
6. Bullock MR, Chesnut RM, Ghajar JM, Gordon DM, Hartl RM, Newell DW. Surgical management of acute subdural hematomas. Neurosurgery. 2006. 58: S16-24 discussion Si-iv
7. Carlsen JG, Cortnum S, Sørensen JC. Recurrence of chronic subdural haematomata with and without post-operative drainage. Br J Neurosurg. 2011. 25: 388-90
8. Catapano JS, Nguyen CL, Wakim AA, Albuquerque FC, Ducruet AF. Middle meningeal artery embolization for chronic subdural hematoma. Front Neurol. 2020. 11: 557233
9. Désir LL, D’Amico R, Link T, Silva D, Ellis JA, Doron O. Middle meningeal artery embolization and the treatment of a chronic subdural hematoma. Cureus. 2021. 13: e18868
10. Edlmann E, Giorgi-Coll S, Whitfield PC, Carpenter KL, Hutchinson PJ. Pathophysiology of chronic subdural haematoma: Inflammation, angiogenesis and implications for pharmacotherapy. J Neuroinflammation. 2017. 14: 108
11. Estcourt LJ, Birchall J, Allard S, Bassey SJ, Hersey P, Kerr JP. Guidelines for the use of platelet transfusions. Br J Haematol. 2017. 176: 365-94
12. Estcourt LJ, Stanworth SJ, Murphy MF. Platelet transfusions for patients with haematological malignancies: Who needs them?. Br J Haematol. 2011. 154: 425-40
13. Feghali J, Yang W, Huang J. Updates in chronic subdural hematoma: Epidemiology, etiology, pathogenesis, treatment, and outcome. World Neurosurg. 2020. 141: 339-45
14. Fiorella D, Arthur AS. Middle meningeal artery embolization for the management of chronic subdural hematoma. J NeuroInterv Surg. 2019. 11: 912-5
15. Gao C, Wang J, Li Y, Zhao H, Li R, Hou L. Incidence and risk of hematologic toxicities with hypomethylating agents in the treatment of myelodysplastic syndromes and acute myeloid leukopenia: A systematic review and meta-analysis. Medicine. 2018. 97: e11860
16. Ghaith AK, El Naamani K, Mualem W, Ghanem M, Rajjoub R, Sweid A. Transradial versus transfemoral approaches in diagnostic and therapeutic neuroendovascular interventions: A meta-analysis of current literature. World Neurosurg. 2022. 164: e694-705
17. Hirano A, Ueoka H. Successful treatment of idiopathic thrombocytopenic purpura by Chinese herbal medicine EK-49 and ascorbic acid in an elderly patient developing chronic subdural hematoma. Geriatr Gerontol Int. 2007. 7: 83-8
18. Holl DC, Volovici V, Dirven CM, Peul WC, van Kooten F, Jellema K. Pathophysiology and nonsurgical treatment of chronic subdural hematoma: From past to present to future. World Neurosurg. 2018. 116: 402-11.e2
19. Hubbard ZS, Al Kasab S, Porto GB, Spiotta A. Chronic subdural hematoma recurrence due to contralateral neovascularization following middle meningeal artery embolization. Interv Neuroradiol. 2021. 28: 639-43
20. Jourdan E, Dombret H, Glaisner S, Micléa JM, Castaigne S, Degos L. Unexpected high incidence of intracranial subdural haematoma during intensive chemotherapy for acute myeloid leukaemia with a monoblastic component. Br J Haematol. 1995. 89: 527-30
21. Jumah F, Osama M, Islim AI, Jumah A, Patra DP, Kosty J. Efficacy and safety of middle meningeal artery embolization in the management of refractory or chronic subdural hematomas: A systematic review and meta-analysis. Acta Neurochir (Wien). 2020. 162: 499-507
22. Kaufman RM, Djulbegovic B, Gernsheimer T, Kleinman S, Tinmouth AT, Capocelli KE. Platelet transfusion: A clinical practice guideline from the AABB. Ann Intern Med. 2015. 162: 205-13
23. Ko BS, Lee JK, Seo BR, Moon SJ, Kim JH, Kim SH. Clinical analysis of risk factors related to recurrent chronic subdural hematoma. J Korean Neurosurg Soc. 2008. 43: 11-5
24. Kolluri SV, Reddy RD, Reddy KP, Naidu MR, Kumari SC. Subdural hematoma secondary to immune thrombocytopenic purpura: Case report. Neurosurgery. 1986. 19: 635-6
25. Kutty RK, Peethambaran AK, Sunilkumar , Anilkumar M. Conservative treatment of chronic subdural hematoma in HIV-associated thrombocytopenia with tranexamic acid. J Int Assoc Provid AIDS Care. 2017. 16: 211-4
26. Leader A, Hofstetter L, Spectre G. Challenges and advances in managing thrombocytopenic cancer patients. J Clin Med. 2021. 10: 1169
27. Lee S, Srivatsan A, Srinivasan VM, Chen SR, Burkhardt JK, Johnson JN. Middle meningeal artery embolization for chronic subdural hematoma in cancer patients with refractory thrombocytopenia. J Neurosurg. 2021. 136: 1273-7
28. Link TW, Boddu S, Marcus J, Rapoport BI, Lavi E, Knopman J. Middle meningeal artery embolization as treatment for chronic subdural hematoma: A case series. Operat Neurosurg. 2018. 14: 556-62
29. Mathews MS, Yu W, Chappell ET. Spontaneous subdural hematoma in the setting of immune thrombocytopenia complicated by ischemic infarcts. Neuroradiol J. 2007. 20: 224-7
30. Moshayedi P, Liebeskind DS. Middle meningeal artery embolization in chronic subdural hematoma: Implications of pathophysiology in trial design. Front Neurol. 2020. 11: 923
31. Mureb MC, Kondziolka D, Shapiro M, Raz E, Nossek E, Haynes J. DynaCT enhancement of subdural membranes after middle meningeal artery embolization: Insights into pathophysiology. World Neurosurg. 2020. 139: e265-70
32. Nam HH, Jang DK, Cho BR. Complications and risk factors after digital subtraction angiography: 1-year single-center study. J Cerebrovasc Endovasc Neurosurg. 2022. 24: 335-40
33. Nia AM, Srinivasan VM, Lall RR, Kan P. Middle meningeal artery embolization for chronic subdural hematoma: A national database study of 191 patients in the United States. World Neurosurg. 2021. 153: e300-7
34. NICE. Overview Blood Transfusion Guidance. Available from: https://www.nice.org.uk/guidance/ng24 [Last accessed on 2022 Mar 02].
35. Rauhala M, Luoto TM, Huhtala H, Iverson GL, Niskakangas T, Öhman J. The incidence of chronic subdural hematomas from 1990 to 2015 in a defined Finnish population. J Neurosurg. 2019. 132: 1147-57
36. Schiffer CA, Bohlke K, Delaney M, Hume H, Magdalinski AJ, McCullough JJ. Platelet transfusion for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2018. 36: 283-99
37. Shen J, Karki M, Jiang T, Zhao B. Complications associated with diagnostic cerebral angiography: A retrospective analysis of 644 consecutive cerebral angiographic cases. Neurol India. 2018. 66: 1154-8
38. Sirh S, Park HR, Park SQ. Usefulness of middle meningeal embolization to prevent recurrent spontaneous chronic subdural hemorrhage. J Cerebrovasc Endovasc Neurosurg. 2018. 20: 40-6
39. Srivatsan A, Mohanty A, Nascimento FA, Hafeez MU, Srinivasan VM, Thomas A. Middle meningeal artery embolization for chronic subdural hematoma: Meta-analysis and systematic review. World Neurosurg. 2019. 122: 613-9
40. Takase H, Tatezuki J, Ikegaya N, Yamamoto D, Hashimoto M, Takagi M. Therapeutic suggestions for chronic subdural hematoma associated with idiopathic thrombocytopenic purpura: A case report and literature review. NMC Case Rep J. 2015. 2: 118-22
41. Tanaka T, Fujimoto S, Saitoh K, Satoh S, Nagamatsu K, Midorikawa H. Superselective angiographic findings of ipsilateral middle meningeal artery of chronic subdural hematoma in adults. No Shinkei Geka. 1998. 26: 339-47
42. Tanaka T, Kaimori M. Histological study of vascular structure between the Dura mater and the outer membrane in chronic subdural hematoma in an adult. No Shinkei Geka. 1999. 27: 431-6
43. Yhim HY, Kim HS, Lee NR, Song EK, Kwak JY, Yim CY. Bilateral subdural hemorrhage as a serious adverse event of dasatinib in a patient with Philadelphia chromosome-positive acute lymphoblastic leukemia. Int J Hematol. 2012. 95: 585-7