- Department of Neurosurgery, St. Marianna University School of Medicine, Toyoko Hospital, Kawasaki, Kanagawa, Japan
- Department of Neurosurgery, St. Marianna University School of Medicine, Kawasaki, Kanagawa, Japan
Correspondence Address:
Hajime Ono
Department of Neurosurgery, St. Marianna University School of Medicine, Kawasaki, Kanagawa, Japan
DOI:10.4103/sni.sni_235_17
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Hajime Ono, Taigen Sase, Hiroshi Takasuna, Yuichiro Tanaka. Mild hemophilia A presaged by recurrent postoperative hemorrhagic complications in an elderly patient. 06-Sep-2017;8:205
How to cite this URL: Hajime Ono, Taigen Sase, Hiroshi Takasuna, Yuichiro Tanaka. Mild hemophilia A presaged by recurrent postoperative hemorrhagic complications in an elderly patient. 06-Sep-2017;8:205. Available from: http://surgicalneurologyint.com/surgicalint-articles/mild-hemophilia-a-presaged-by-recurrent-postoperative-hemorrhagic-complications-in-an-elderly-patient/
Abstract
Background:Mild hemophilia without spontaneous bleeding can remain undiagnosed for a lifetime. However, intracranial hemorrhage is one of the most serious complications for patients with hemophilia. In addition, hemorrhagic complications after emergency surgery tend to arise from coagulopathy.
Case Description:An 80-year-old man was admitted with left hemiparesis and disturbed consciousness. He had no history of trauma, fever, or drug and alcohol intake. Computed tomography imaging upon admission disclosed a hemispheric subdural hematoma with a midline shift. No vascular abnormalities were identified as a source of the hemorrhage. The hematoma was removed on an emergency basis with external decompression. However, a large subcutaneous hematoma was again evident on the following day. Insufficient hemostatic maneuvers during surgery were considered the cause of this hemorrhagic complication. A second operation was performed to achieve hemostasis of the subcutaneous and muscle tissue. Thereafter, he was rehabilitated without treatment for hemophilia as he had no bleeding episodes. Cranioplasty proceeded using artificial bone at 40 days after the first operation. However, epidural hematoma developed again on postoperative day 1. His neurological status did not worsen so a repeat procedure was unnecessary. Close scrutiny uncovered a diagnosis of mild hemophilia A.
Conclusions:Accurate diagnosis is important for the management of postoperative hemorrhagic complications caused by pathologies of the coagulation system. Sufficient hemostasis of hemorrhage from subcutaneous and muscle tissue is essential even during emergency surgery to avoid postoperative complications. A diagnosis of hemophilia should be considered in the face of prolonged activated partial thromboplastin time (APTT).
Keywords: Activated partial thromboplastin time, acute subdural hematoma, diagnosis mild hemophilia A, postoperative complication
INTRODUCTION
The incidence of hemorrhagic complications after neurosurgical surgery has declined due to advances in surgical procedures and medical instruments. However, the postoperative hemorrhagic complication rate increases in the presence of coagulopathies including hemophilia. Hemophilia is usually diagnosed during childhood because bleeding episodes usually present before the age of 5 years. Therefore, mild hemophilia may be asymptomatic and diagnosed incidentally at the time of surgery or trauma, which can result in serious complications. Intracranial hemorrhage can occur in adults with or without a diagnosis of hemophilia.[
CASE PRESENTATION
An 80-year-old man was admitted for left hemiparesis and disturbed consciousness at his workplace early in the morning. His medical history contained no head injuries, drug use, malignancies, blood diseases, or autoimmune diseases. He had never experienced bleeding episodes and his family history was unremarkable. His vital signs upon admission were blood pressure, 150/78 mmHg; heart rate, 105 bpm; respiration rate, 17 breaths/min; temperature, 36.7°C; and oxygen saturation, 97% on room air. Laboratory findings revealed a prolonged APTT of 39.8 (normal range, 25–35) s, but a normal prothrombin time of 92.9% (normal range, 75–125%). Other values within normal ranges included platelet count of 186 × 103 (normal range, 152–382) × 103/μL; hemoglobin, 14.9 (normal range, 14.0–17.0) g/dL; and hematocrit, 44.6% (normal range, 43.0–51.0%).
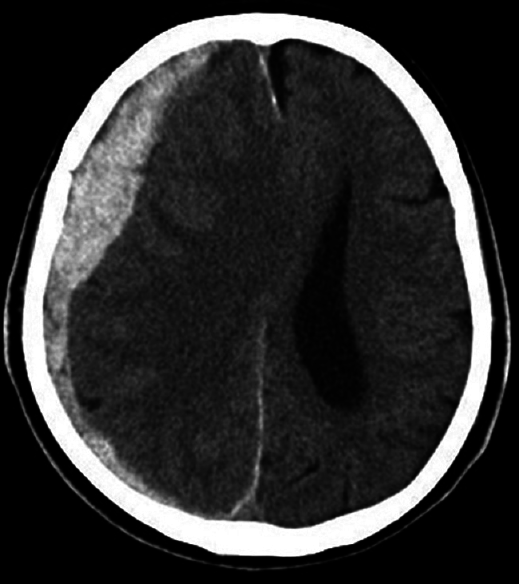
Vital abnormalities were not evident at the time of presentation, however, neurological findings showed left hemiparesis including the face. Computed tomography (CT) imaging upon admission showed right thick ASDH with median deviation [
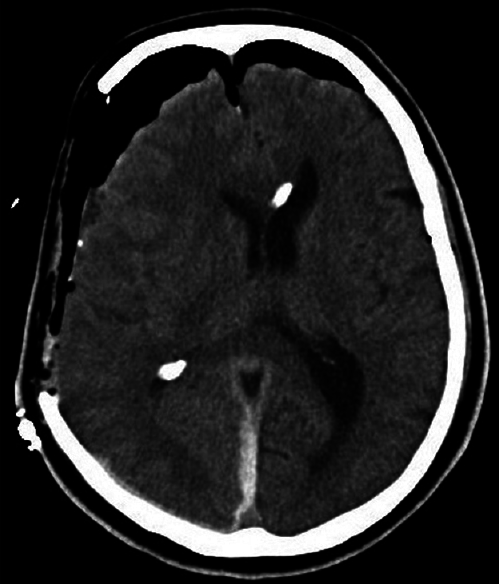
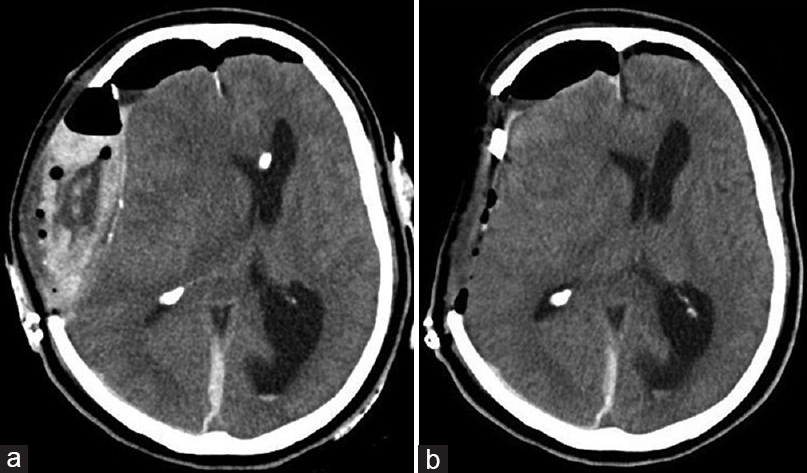
Postoperative CT showed a small amount of hematoma and improved median deviation [
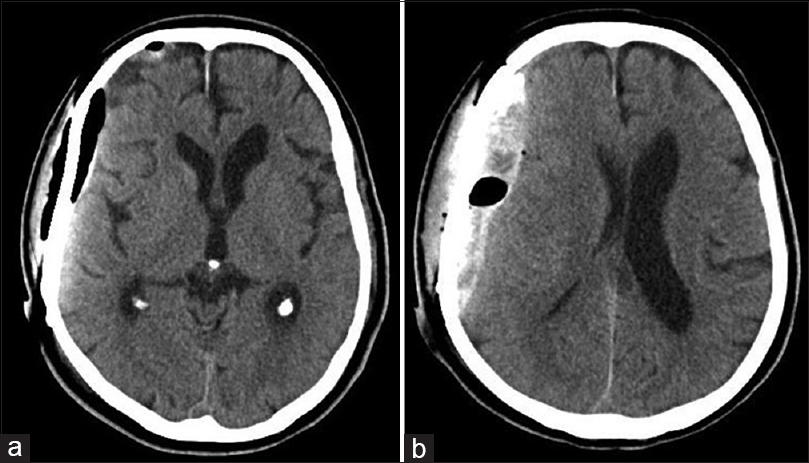
Cranioplasty proceeded when his general condition was stabilized at 40 days after hospitalization, although the APTT remained slightly prolonged at 35.2 s. CT imaging showed a small amount of hematoma and air immediately after the cranioplasty [
DISCUSSION
The current frequency of intracranial hemorrhage in patients with hemorrhagic disease caused by the lack of factor VIII (hemophilia A) or factor IX (hemophilia B) ranges 2.7–11.2%.[
Hemophilia is classified as mild, moderate, or severe according to whether factor VIII levels are 6–25%, 2–5%, or <1%, respectively.[
Our patient did not develop bleeding symptoms due to trauma or surgery; the diagnosis of mild hemophilia was derived from scrutiny of the coagulation system. The possibility of hemophilia in ASDH without trauma history should be considered. Our patient required emergency surgery for ASDH, but the diagnosis of hemophilia was delayed because of the following reasons: first, the cause of rebleeding after the initial emergency procedure had to be evaluated, and second, the underlying cause of the mild prolonged APTT had to be determined.
Postoperative hemorrhagic complications after neurological surgery should be considered. The clinical rate of deterioration is about 0.77–6.9% among patients with postoperative hematoma complications.[
Desai noted that 1.9% of postoperative hemorrhage requires reoperation for intracranial pressure control (10). Furthermore, coagulopathy might increase postoperative bleeding if the cause of ASDH is traumatic accidents.[
The massive hematoma arising from subcutaneous and muscle tissue in our patient after the first surgical procedure was associated with a cerebral hernia. Therefore, reoperation was necessary to control intracranial pressure and prevent rebleeding. We initially considered that inadequate surgical technique and procedures related to hemostasis after the initial emergency surgery caused the hematoma.[
We investigated the status of APTT and the clinical course of patients with mild hemophilia A. The initial APTT in our patient after craniotomy was mildly prolonged at 39.8 s, but the change in APTT did not parallel the clinical hemorrhagic symptoms. Some reports describe patients with mildly prolonged or normal APTT.[
The measured value should be carefully considered because various causes could prolong the APTT including the current status of the patient and the anticipated clinical course.[
Stieltjes et al. found that 50% of cerebral hemorrhagic episodes occurred in patients aged >15 years with mild hemophilia, and about 33% of cerebral hemorrhages occurred in patents with moderate or mild hemophilia.[
The general incidence of cerebral hemorrhage is higher in the elderly than in young persons, and hypertension is considered a risk.[
Our experience with the present patient has highlighted an important consideration: coagulation should be appropriately assessed if postoperative hemorrhage occurs in an emergency situation even when surgical technique is appropriate. Thus, hemophilia and other diseases associated with blood coagulation factors can be diagnosed.
CONCLUSIONS
Abnormal bleeding can develop in patients with mild hemophilia because of surgery or tooth removal, but rarely during activities of daily living. Therefore, mild hemophilia determined only by a general blood coagulation examination and medical practice is insufficient. That is, neurosurgeons should become more aware of surgical treatment for blood coagulopathies including hemophilia regardless of age.
Consent/assent
The patient provided written, informed consent/assent to the publication of this case report.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors have no conflicts of interest concerning the materials or methods used in this study or the findings described herein.
References
1. Allard CB, Scarpelini S, Rhind SG, Baker AJ, Shek PN, Tien H. Abnormal coagulation tests are associated with progression of traumatic intracranial hemorrhage. J Trauma. 2009. 67: 959-67
2. Angelini D, Konkle BA, Sood SL. Aging among persons with hemophilia: Contemporary concerns. Semin Hematol. 2016. 53: 35-9
3. Antunes SV, Vicari P, Cavalheiro S, Bordin JO. Intracranial haemorrhage among a population of haemophilic patients in Brazil. Haemophilia. 2003. 9: 573-7
4. Basali A, Mascha EJ, Kalfas I, Schubert A. Relation between perioperative hypertension and intracranial hemorrhage after craniotomy. Anesthesiology. 2000. 93: 48-54
5. Bray GL, Luban NL. Hemophilia presenting with intracranial hemorrhage. An approach to the infant with intracranial bleeding and coagulopathy. Am J Dis Child. 1987. 141: 1215-7
6. Bullock R, Hannemann CO, Murray L, Teasdale GM. Recurrent hematomas following craniotomy for traumatic intracranial mass. J Neurosurg. 1990. 72: 9-14
7. Canaro M, Goranova-Marinova V, Berntorp E. The ageing patient with haemophilia. Eur J Haematol. 2015. 77: 17-22
8. Chan K, Mann K, Chan T. The significance of thrombocytopenia in the development of postoperative intracranial haematoma. J Neurosurg. 1989. 71: 38-41
9. Chorba TL, Holman RC, Strive TW, Clarke MJ, Evatt BL. Changes in longevity and causes of death among persons with hemophilia. Am J Hematol. 1994. 45: 112-21
10. Desai VR, Grossman R, Sparrow H. Incidence of Intracranial Hemorrhage After a Cranial Operation. Cureus. 2016. 20: e616-
11. Desai VR, Scranton RA, Britz GW. Management of Recurrent Subdural Hematomas. Neurosurg Clin N Am. 2017. 28: 279-86
12. DiMichele D, Neufeld EJ. Hemophilia. A new approach to an old disease. Hematol Oncol Clin North Am. 1998. 12: 1315-44
13. Eyster ME, Gill FM, Blatt PM, Hilgartner MW, Ballard JO, Kinney TR. Central nervous system bleeding in hemophiliacs. Blood. 1978. 51: 1179-88
14. Franchini M, Favaloro EJ, Lippi G. Mild hemophilia A. J Thromb Haemost. 2010. 8: 421-32
15. Franchini M, Mannucci PM. Acquired haemophilia A: A 2013 update. Thromb Haemost. 2013. 110: 1114-20
16. Gerlach R, Tolle F, Raabe A, Zimmermann M, Siegemund A, Seifert V. Increased risk for postoperative hemorrhage after intracranial surgery in patients with decreased factor XIII activity: Implications of a prospective study. Stroke. 2002. 33: 1618-23
17. Hermans C, de Moerloose P, Dolan G. Clinical management of older persons with haemophilia. Crit Rev Oncol Hematol. 2014. 89: 197-206
18. Kalfas IH, Little JR. Postoperative hemorrhage: A survey of 4992 intracranial procedures. Neurosurgery. 1988. 23: 343-7
19. Klinge J, Auberger K, Auerswald G, Brackmann HH, Mauz-Körholz C, Kreuz W. Prevalence and outcome of intracranial haemorrhage in haemophiliacs—a survey of the paediatric group of the German Society of Thrombosis and Haemostasis (GHT). Eur J Pediatr. 1999. 158: 162-5
20. Konkle BA. Clinical challenges within the aging hemophilia population. Thromb Res. 2011. 127: S10-3
21. Ljung RC. Intracranial haemorrhage in haemophilia A and B. Br J Haematol. 2008. 140: 378-84
22. Myles LM, Massicotte P, Drake J. Intracranial hemorrhage in neonates with unrecognized hemophilia A: A persisting problem. Pediatr Neurosurg. 2001. 34: 94-7
23. Nelson MD, Maeder MA, Usner D, Mitchell WG, Fenstermacher MJ, Wilson DA. Prevalence and incidence of intracranial haemorrhage in a population of children with hemophilia. Hemophilia. 1999. 5: 306-12
24. Nuss R, Soucie JM, Evatt B. Hemophilia Surveillance System Project Investigators. Changes in the occurrence of and risk factors for hemophilia-associated intracranial hemorrhage. Am J Hematol. 2001. 68: 37-42
25. Philipp C. The aging patient with hemophilia: Complications, comorbidities, and management issues. Hematology Am Soc Hematol Educ Program. 2010. 10: 191-6
26. Reding MT, Cooper DL. Barriers to effective diagnosis and management of a bleeding patient with undiagnosed bleeding disorder across multiple specialties: Results of a quantitative case-based survey. J Multidiscrip Healthc. 2012. 5: 277-87
27. Shetty S, Bhave M, Ghosh K. Acquired hemophilia A: Diagnosis, aetiology, clinical spectrum and treatment options. Autoimmun Rev. 2011. 10: 311-6
28. Stieltjes N, Calvez T, Demiguel V, Torchet MF, Briquel ME, Fressinaud E. Intracranial haemorrhages in French haemophilia patients (1991–2001): Clinical presentation, management and prognosis factors for death. Haemophilia. 2005. 11: 452-8
29. Tiede A, Werwitzke S, Scharf RE. Laboratory diagnosis of acquired hemophilia A: Limitations, consequences, and challenges. Semin Thromb Hemost. 2014. 40: 803-11
30. Triemstra M, Rosendaal FR, Smit C, Van der Ploeg HM, Briët E. Mortality in patients with hemophilia. Changes in Dutch population from 1986 to 1992 and 1973 to 1986. Ann Intern Med. 1995. 123: 823-87
31. Tsuyama N, Ichiba T, Naito H. Unusual Initial Manifestation of Acquired Hemophilia A: A Normal Activated Partial Thromboplastin Time, Intramuscular Hematoma and Cerebral Hemorrhage. Intern Med. 2016. 55: 3347-9
32. Zanon E, Iorio A, Rocino A, Artoni A, Santoro R, Tagliaferri A. Intracranial haemorrhage in the Italian population of haemophilia patients with and without inhibitors. Haemophilia. 2012. 18: 39-45