- Department of Surgery, Division of Neurosurgery, Chiang Mai University, Chiang Mai, Thailand.
Correspondence Address:
Chumpon Jetjumnong, Department of Surgery, Division of Neurosurgery, Chiang Mai University, Chiang Mai, Thailand.
DOI:10.25259/SNI_844_2021
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Chumpon Jetjumnong, Thunya Norasetthada. Modified McKenzie-Dandy operation for a cervical dystonia patient who failed selective peripheral denervation: A case report and literature review. 29-Jan-2022;13:31
How to cite this URL: Chumpon Jetjumnong, Thunya Norasetthada. Modified McKenzie-Dandy operation for a cervical dystonia patient who failed selective peripheral denervation: A case report and literature review. 29-Jan-2022;13:31. Available from: https://surgicalneurologyint.com/surgicalint-articles/11365/
Abstract
Background: Cervical dystonia (CD) is a rare and difficult-to-treat disorder. Various neurosurgical options are available, each with its own set of advantages and disadvantages. We investigated using the modified McKenzie-Dandy operation for a patient with CD who failed selective peripheral denervation (SPD).
Case Description: A 42-year-old man presented left-sided rotational torticollis for 3 years. He was referred for surgery after treating with a variety of oral medications and repeated botulinum toxin injections that became ineffective. For the first operation, the patient underwent SPD (modified Bertrand’s operation); unfortunately, the postoperative outcome was unsatisfactory, and the operation was considered a failure. After his symptoms did not improve after 6 months, the modified McKenzie-Dandy operation was performed. Immediately following surgery, he experienced satisfactory outcomes. He was able to resume his normal activities and employment after 1 month after recovering from his temporary swallowing difficulties. He only complained of minor neck pain and no recurrence was observed after 3 years follow-up.
Conclusion: For patients who have failed SPD, a modified McKenzie-Dandy procedure is a feasible and effective option. The procedure is relatively safe when performed properly, and the long-term effects can be maintained.
Keywords: Cervical dystonia, Denervation, Rhizotomy, Spasmodic torticollis
INTRODUCTION
Cervical dystonia (CD) is an uncommon movement disorder characterized by involuntary intermittent twisting or sustained contractions of many cervical muscle groups. The various forms of disfiguring head-and-neck postures that are caused by imbalanced action of several paired cervical muscles include rotational torticollis (also known as spasmodic torticollis; ST), laterocollis, anterocollis, retrocollis, or their various combinations.[
Among the various surgical options, most neurosurgeons prefer extraspinal selective peripheral denervation (SPD), widely known as Bertrand’s operation, or its modifications.[
The authors have described a case in which SPD failed to provide significant symptomatic relief, necessitating an intradural rhizotomy procedure (modified McKenzie-Dandy operation)[
CASE DESCRIPTION
Case history and physical examination
A 42-year-old man presented to our department after 3 years after receiving a diagnosis of the left-sided rotational torticollis. He began to exhibit symptoms 2 months after a motorcycle accident in which he temporarily lost consciousness and experienced minor neck pain. Initially, he exhibited a paroxysmal involuntarily head rotation toward the left without elevation of the shoulder. No other symptoms were evident such as blepharospasm, oromandibular dyskinesia, or tremor. The symptom was exacerbated by anxiety or mental stress. Later, the head rotation became more tonic, resulting in a constant fixed rotational position except when sleeping. He had to touch his chin to keep his head in the midline. No history of torticollis or dystonia was reported among his family members. Treatments with a variety of oral medications as well as repeated botulinum toxin injections were no longer effective. His symptoms deteriorated and became more uncomfortable, limiting his daily activities and ability to work. He was then referred to our department for surgical consultation.
The neurological examination results were normal at the time of admission, except for palpable abnormal sustained contractions of the right sternocleidomastoid (SCM) and left posterior neck muscles (splenius capitis and semi-spinalis capitis). A sensory tick (geste antagonistique) was noticed when he touched his chin. The Toronto Western ST Rating Scale (TWSTRS) gave a score of 24 on the torticollis severity scale, 23 on the disability scale, and 11 on the pain scale (total score = 58). An electromyography (EMG) was not used to record aberrant muscle activation. His magnetic resonance imaging (MRI) brain revealed no abnormalities, but his MRI spine indicated cervical spondylosis with mild narrowing of the left neural foramen at C3 to C4 and C6 to C7 levels.
Following a thorough discussion of the risks and benefits of surgical treatment, the patient opted to undergo the operation in February 2018. We used the modified Bertrand’s procedure to denervate the left posterior neck muscles, in which we strictly followed the technique described by Taira et al.[
Unfortunately, after the operation, the patient had no meaningful relief, with a TWSTRS score of 22, 23, and 9 on the torticollis severity scale, disability scale, and pain scale, respectively (total score = 54). After 6 months of intensive exercise and physiotherapy, the symptoms appeared to be unchanged. A second stage operation with modified McKenzie-Dandy operation was considered and performed in September 2018.
Surgical procedure
The patient was placed in the prone position. We used spinal somatosensory evoked potential and motor evoked potential for neuromonitoring. An EMG was used to monitor the SCM and trapezius muscle activities. A left-sided Bertrand’s hockey stick incision was made. The trapezius, splenius capitis, and semi-spinalis muscles were dissected and sectioned from their insertion just below the nuchal line of the occipital bone. Subperiosteal dissection was then performed bilaterally. At the C1 and C2 spinous processes, the left inferior oblique capitis and rectus capitis major and minor were detached and severed from their origin. A laminectomy of C1 to C3 was carried out and the posterior rim of the foramen magnum was removed. The dura was then opened to expose the lower brainstem and upper cervical spinal cord. Using the microsurgical technique, an arachnoid dissection was performed. After cutting the denticulate ligaments, we used bipolar electrode stimulation (0.1–0.3 volts) to detect the anterior cervical nerve rootlets. The first cervical motor root was identified as it runs transversely just below the proximal intradural part of the vertebral artery. Some of the left anterior C1 and C2 rootlets were already cut by the previous operation. The left anterior C1 to C4 rhizotomies were carried out using bipolar and microscissors. The right anterior C1 to C3 rootlets, on the other hand, were sectioned less aggressively to minimize the risk of swallowing difficulty and bilateral phrenic nerve impairment. Any tiny blood vessels were carefully preserved. The spinal root of the right SAN was tracked upward until where it crosses the vertebral artery. Neither abnormal vascular loop nor the connection between the SAN and the C1 root (McKenzie branch) was found. Three to four spinal rootlets of the SAN that produced a strong contraction of the SCM muscle on electrical stimulation were sectioned, while the upper medullary rootlets were preserved to minimize the risk to the pharynx or vocal cord impairment [
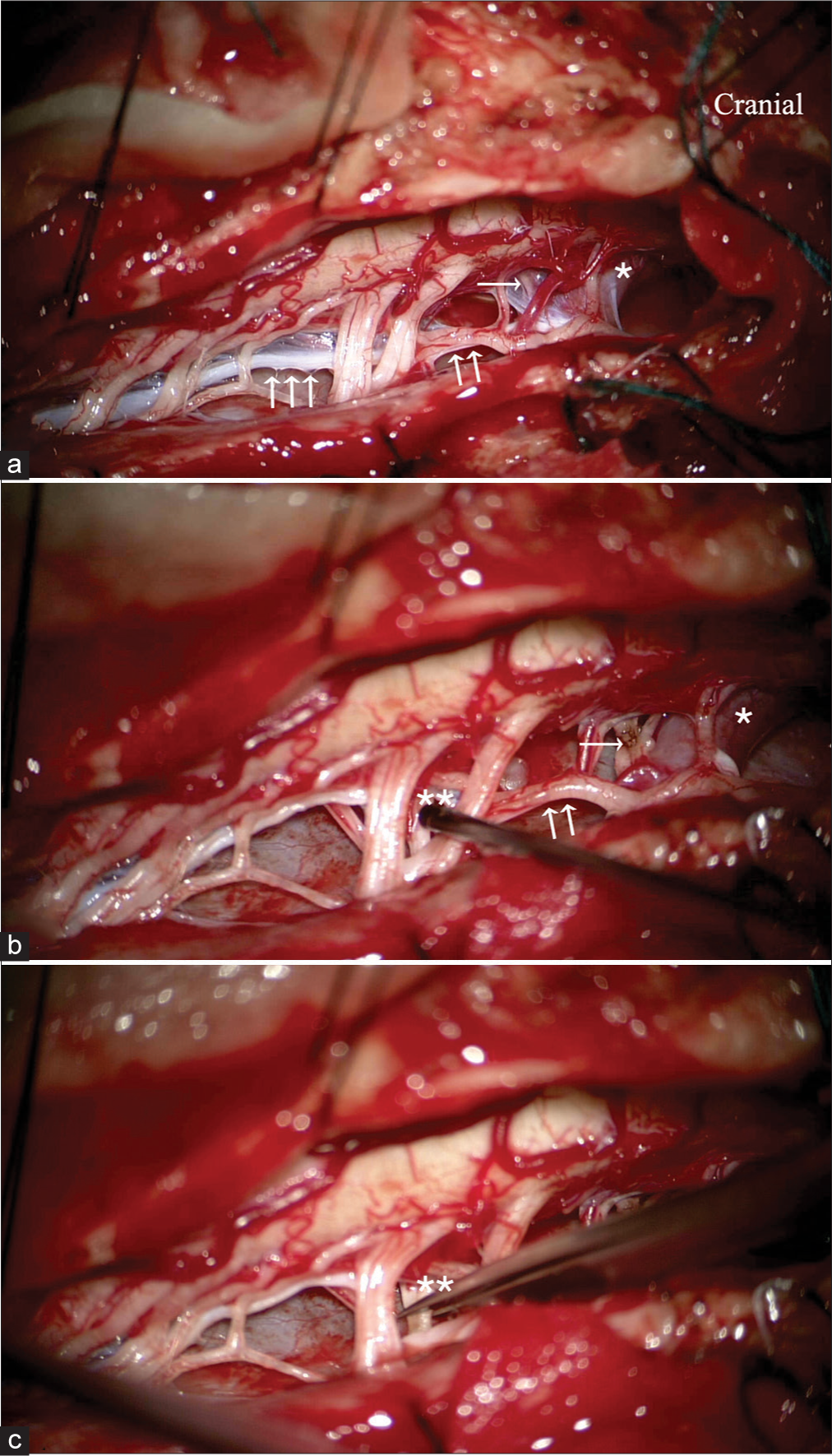
Figure 1:
Intraoperative photograph showing the anatomy after opening the dura (a and b). The right-sided spinal accessory nerve; SAN (double arrow) runs parallel to the spinal cord. The denticulate ligaments (triple arrow) were carefully dissected. The anterior root of C-1 (single arrow) is often hidden just beneath the vertebral artery (asterisk). The McKenzie nerve was not identified in this case. The C1-C3 and SAN motor rootlets (double asterisk) were sectioned selectively, using electrical stimulation (c).
Postoperative course
After the operation, the patient’s head and neck returned to normal posture. He stated that he had a marked improvement. He complained of a mild degree of swallow difficulty. He did not have choking or dysphagia. The symptoms were completely resolved 1 month after surgery. The TWSTRS at 3 months gave a score of 3 on the torticollis severity scale, 4 on the disability scale, and 2 on the pain Scale (total score = 9). He could return to his previous job and social activities. He experienced no numbness or dysesthesia in the occipital area. At 3 years follow-up, he was free of symptoms, and the effect of treatment was still sustained [
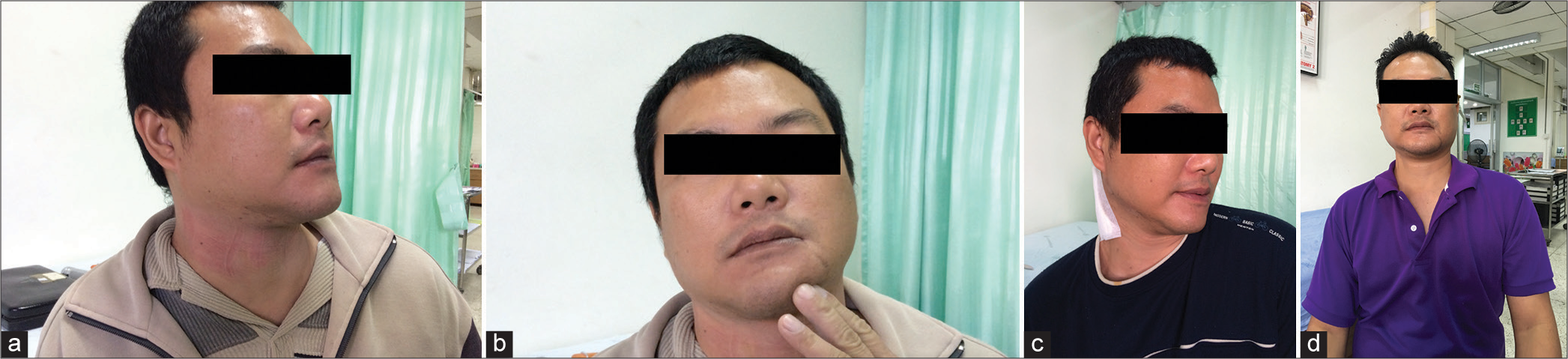
Figure 2:
(a) The patient’s posture at the time of presentation. (b) When he touched his chin, sensory trick was observed. (c) After the patient underwent the selective peripheral denervation operation, his symptoms persisted. (d) After reoperation with the modified McKenzie-Dandy operation, a favorable result was obtained.
DISCUSSION
Nonoperative treatments for CD, including oral medications, botulinum toxin injections, and physiotherapies, have been used as first-line treatments, albeit they typically only provide minor and temporary symptom relief. In addition, a significant number of patients failed to respond or became refractory to botulinum toxin after repeated injections, due to developing anti-botulinum toxin antibodies. In such cases, a neurosurgical procedure should be considered.
Bertrand et al. described an extraspinal SPD in the early 1980s, and it has since become the most preferred surgical treatment for CD among neurosurgeons.[
Various steps in SPD can be mistaken or overlooked. Thus, reoperation using the same surgical approach or modifications can be considered as the first option. However, reoperation has several disadvantages, including the difficulty of searching for small residual nerve branches in fibrotic or scar tissue, as well as, the loss of the original anatomical plane, which makes dissection difficult, both of which lead to reoperation failure. Furthermore, an extensive dissection can also cause unintentional damage to the arteries or nerves that supply other muscles in the neck or shoulder, particularly the branches to the trapezius.
McKenzie originally performed intradural sectioning of the anterior and posterior roots of the upper cervical spine, as well as partial sectioning of the SAN, in treating patients with CD.[
In this case, we modified the McKenzie-Dandy operation by performing an asymmetrical upper anterior rhizotomy (C1 to C3 ± C4) and selective sectioning of the SAN’s rootlets, intradurally. The aim was to interrupt all motor supplies to the muscles that controlled head rotation while minimizing the risk of swallowing difficulties and trapezius paralysis. We used Bertrand’s hockey stick incision to increase surgical exposure and created a myotomy effect by sectioning and dissecting the trapezius and splenius capitis from their insertion directly below the occipital bone.[
Several issues in this operation should be considered. The caudal limit of intradural anterior rhizotomy involves unilateral C4 to prevent the risk of bilateral phrenic nerve paralysis and the motor roots that supply the brachial plexus. The superior rootlets, or cranial root, of intradural SAN should always be preserved because they form a part of the inferior vagal nerve rootlets that are responsible for pharyngeal and vocal cord function.[
There have been reports of other surgical procedures for patients who have failed SPD. Globus pallidus deep brain stimulation (GPi-DBS) has become more commonly used in recent years, and some surgeons now advocate it as a first-line treatment for CD rather than denervation surgery, especially in complex forms of CD.[
CONCLUSION
The modified McKenzie-Dandy surgery was a feasible and effective alternative for patients who have failed SPD or Bertrand’s operation. When performed properly, it remains relatively safe, and its long-term effects can be maintained.
Declaration of patient consent
Institutional Review Board permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Aljuboori Z, Ball T, Nauta H. Modified McKenzie procedure for the treatment of fixed painful torticollis. Neurosurg Focus Video. 2020. 3: 1-3
2. Anderson WS, Lawson HC, Belzberg AJ, Lenz FA. Selective denervation of the levator scapulae muscle: An amendment to the Bertrand procedure for the treatment of spasmodic torticollis. J Neurosurg. 2008. 108: 757-63
3. Bergenheim AT, Nordh E, Larsson E, Hariz MI. Selective peripheral denervation for cervical dystonia: Long-term follow-up. J Neurol Neurosurg Psychiatry. 2015. 86: 1307-13
4. Bertrand C, Negro PM, Martinez SN. Technical aspects of selective peripheral denervation for spasmodic torticollis. Appl Neurophysiol. 1982. 45: 326-30
5. Bertrand CM. Selective peripheral denervation for spasmodic torticollis: Surgical technique, results, and observations in 260 cases. Surg Neurol. 1993. 40: 96-103
6. Braun V, Richter HP. Selective peripheral denervation for spasmodic torticollis: 13-year experience with 155 patients. J Neurosurg. 2002. 97: 207-12
7. Brin MF, Benabou R. Cervical dystonia (torticollis). Curr Treat Options Neurol. 1999. 1: 33-43
8. Chen X, Ma A, Liang J, Ji S, Pei S. Selective denervation and resection of cervical muscles in the treatment of spasmodic torticollis: Long-term follow-up results in 207 cases. Stereotact Funct Neurosurg. 2000. 75: 96-102
9. Chung M, Han I, Chung SS, Jang DK, Huh R. Effectiveness of selective peripheral denervation in combination with pallidal deep brain stimulation for the treatment of cervical dystonia. Acta Neurochir (Wien). 2015. 157: 435-42
10. Cohen-Gadol AA, Ahlskog JE, Matsumoto JY, Swenson MA, McClelland RL, Davis DH. Selective peripheral denervation for the treatment of intractable spasmodic torticollis: Experience with 168 patients at the Mayo Clinic. J Neurosurg. 2003. 98: 1247-54
11. Contarino MF, van den Munckhof P, Tijssen MA, de Bie RM, Bosch DA, Schuurman PR. Selective peripheral denervation: Comparison with pallidal stimulation and literature review. J Neurol. 2014. 261: 300-8
12. Dauer WT, Burke RE, Greene P, Fahn S. Current concepts on the clinical features, aetiology and management of idiopathic cervical dystonia. Brain. 1998. 121: 547-60
13. Ford B, Louis ED, Greene P, Fahn S. Outcome of selective ramisectomy for botulinum toxin resistant torticollis. J Neurol Neurosurg Psychiatry. 1998. 65: 472-8
14. Friedman AH, Nashold BS, Sharp R, Caputi F, Arruda J. Treatment of spasmodic torticollis with intradural selective rhizotomies. J Neurosurg. 1993. 78: 46-53
15. Hamby WB, Schiffer S. Spasmodic torticollis: Results after cervical rhizotomy in 50 cases. J Neurosurg. 1969. 31: 323-6
16. Horisawa S, Fukui A, Kohara K, Kawamata T, Taira T. Unilateral pallidotomy in the treatment of cervical dystonia: A retrospective observational study. J Neurosurg. 2021. 134: 216-22
17. Horisawa S, Goto S, Takeda N, Terashima H, Kawamata T, Taira T. Bilateral pallidotomy for cervical dystonia after failed selective peripheral denervation. World Neurosurg. 2016. 89: 728.e1-4
18. Huh R, Chung M. Electrophysiological interpretations of the clinical response to stimulation parameters of pallidal deep brain stimulation for cervical dystonia. Acta Neurochir (Wien). 2016. 158: 2029-38
19. Krauss JK, Toups EG, Jankovic J, Grossman RG. Symptomatic and functional outcome of surgical treatment of cervical dystonia. J Neurol Neurosurg Psychiatry. 1997. 63: 642-8
20. Lai Y, Huang P, Zhang C, Hu L, Deng Z, Li D. Unilateral pallidotomy as a potential rescue therapy for cervical dystonia after unsatisfactory selective peripheral denervation. J Neurosurg Spine. 2020. 33: 658-66
21. Loher TJ, Pohle T, Krauss JK. Functional stereotactic surgery for treatment of cervical dystonia: Review of the experience from the lesional era. Stereotact Funct Neurosurg. 2004. 82: 1-13
22. McKenzie KG. The surgical treatment of spasmodic torticollis. Clin Neurosurg. 1954. 2: 33-43
23. Meyer CH. Outcome of selective peripheral denervation for cervical dystonia. Stereotact Funct Neurosurg. 2001. 77: 44-7
24. Munchau A, Palmer JD, Dressler D, O’Sullivan JD, Tsang KL, Jahanshahi M. Prospective study of selective peripheral denervation for botulinum-toxin resistant patients with cervical dystonia. Brain. 2001. 124: 769-83
25. Oh CS, Chung IH, Lee KS. Topographical anatomy on the communicating branch between the spinal accessory nerve and the anterior root of the first cervical nerve. Surg Radiol Anat. 2003. 25: 207-9
26. Saylam CY, Orhan M, Ikiz Z, Ucerler H, Zileli M. The incidence and anatomical features of the McKenzie branch: A cadaver study. Turk Neurosurg. 2009. 19: 42-4
27. Sorensen BF, Hamby WB. Spasmodic torticollis. Results in 71 surgically treated patients. JAMA. 1965. 194: 706-8
28. Taira T, Hori T. A novel denervation procedure for idiopathic cervical dystonia. Stereotact Funct Neurosurg. 2003. 80: 92-5
29. Taira T, Kobayashi T, Takahashi K, Hori T. A new denervation procedure for idiopathic cervical dystonia. J Neurosurg. 2002. 97: 201-6
30. Tsymbaliuk VI, Tretyak IB, Freidman MY, Gatskiy AA. Denervation and myotomy of muscles of the omotrapezoid triangle of the neck improve the outcomes of surgical treatment of laterocollis and torticollis subtypes of spasmodic torticollis: 58 case analysis. Acta Neurochir (Wien). 2016. 158: 1159-64
31. Tubbs RS, Benninger B, Loukas M, Cohen-Gadol AA. Cranial roots of the accessory nerve exist in the majority of adult humans. Clin Anat. 2014. 27: 102-7
32. Tubbs RS, Benninger B, Loukas M, Cohen-Gadol AA. The nerve of McKenzie: Anatomic study with application to intradural rhizotomy for spasmodic torticollis. Br J Neurosurg. 2014. 28: 650-2
33. Tubbs RS, Loukas M, Yalcin B, Shoja MM, Cohen-Gadol AA. Classification and clinical anatomy of the first spinal nerve: Surgical implications. J Neurosurg Spine. 2009. 10: 390-4
34. Wang J, Li J, Han L, Guo S, Wang L, Xiong Z, Ma J, Liang J, Wang L. Selective peripheral denervation for the treatment of spasmodic torticollis: Long-term follow-up results from 648 patients. Acta Neurochir (Wien). 2015. 157: 427-33