- Department of Neurosurgery, Kitasato University School of Medicine, Sagamihara, Kanagawa, Japan.
- Division of Brain Tumor Translational Research, National Cancer Center Research Institute, Tokyo, Japan.
- Department of Pathology, Public Tomioka General Hospital, Tomioka, Japan.
- Department of Pathology, National Center for Child Health and Development, Tokyo, Japan.
Correspondence Address:
Ichiyo Shibahara, Department of Neurosurgery, Kitasato University School of Medicine, Sagamihara, Kanagawa, Japan.
DOI:10.25259/SNI_55_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Hajime Handa1, Ichiyo Shibahara1, Yoshiko Nakano2, Madoka Inukai1, Sumito Sato1, Takuichiro Hide1, Junko Hirato3, Takako Yoshioka4, Koichi Ichimura2, Toshihiro Kumabe1. Molecular analyses of rosette-forming glioneuronal tumor of the midbrain tegmentum: A report of two cases and a review of the FGFR1 status in unusual tumor locations. 20-May-2022;13:213
How to cite this URL: Hajime Handa1, Ichiyo Shibahara1, Yoshiko Nakano2, Madoka Inukai1, Sumito Sato1, Takuichiro Hide1, Junko Hirato3, Takako Yoshioka4, Koichi Ichimura2, Toshihiro Kumabe1. Molecular analyses of rosette-forming glioneuronal tumor of the midbrain tegmentum: A report of two cases and a review of the FGFR1 status in unusual tumor locations. 20-May-2022;13:213. Available from: https://surgicalneurologyint.com/surgicalint-articles/11602/
Abstract
Background: Rosette-forming glioneuronal tumor (RGNT) is a rare tumor that arises primarily in the posterior fossa, with molecular features of FGFR1 mutation. A previous study reported that brainstem RGNT accounts for only 2.7% cases; therefore, midbrain RGNT is infrequent.
Case Description: The authors encountered two cases of RGNT located in the midbrain tegmentum (Case 1: 23-year-old woman and Case 2: 18-year-old boy), both exhibiting similar cystic components with gadolinium-enhanced cyst walls on preoperative magnetic resonance imaging, surgically resected through the occipital transtentorial approach. Histological findings in both cases comprised two characteristic architectures of neurocytic and glial components, typical of RGNT. Molecular assessment revealed no FGFR1 mutation in the initial specimen, but revealed FGFR1 K656E mutation in the recurrent specimen in Case 1 and showed no FGFR1 mutation but showed TERT C228T mutation in Case 2. Neither case revealed IDH1/2, BRAF, H3F3A K27, H3F3A G34, or HIST1H3B K27 mutations. DNA methylation-based classification (molecularneuropathology.org) categorized both cases as RGNT, whose calibrated scores were 0.99 and 0.47 in Cases 1 and 2, respectively.
Conclusion: Midbrain tegmentum RGNTs exhibited typical histological features but varied FGFR1 statuses with TERT mutation. RGNT in rare locations may carry different molecular alterations than those in other common locations, such as the posterior fossa.
Keywords: FGFR1, Midbrain, Rosette-forming glioneuronal tumor, Tegmentum, TERT
INTRODUCTION
Rosette-forming glioneuronal tumor (RGNT) is a rare tumor that arises primarily in the posterior fossa[
We encountered two cases of RGNT located in the midbrain tegmentum, both of which demonstrated a gadolinium-enhanced cyst wall and were radically resected using the occipital transtentorial approach. Molecular analyses of the rare midbrain tegmentum RGNT included FGFR1, TERT, IDH1, IDH2, H3F3A, HIST1H3B, BRAF, KIAA1549-BRAF fusion, and DNA methylation-based classifiers provided by MolecularNeuropathology.org.[
CASE DESCRIPTION
Clinical summary
Case 1
A 23-year-old woman presented with sensory disturbances in the left face and hand. Gadolinium-enhanced T1-weighted (GdT1WI) magnetic resonance imaging (MRI) revealed enhancement suggestive of a thin cystic wall without nodule formation on the right side of the midbrain tegmentum [
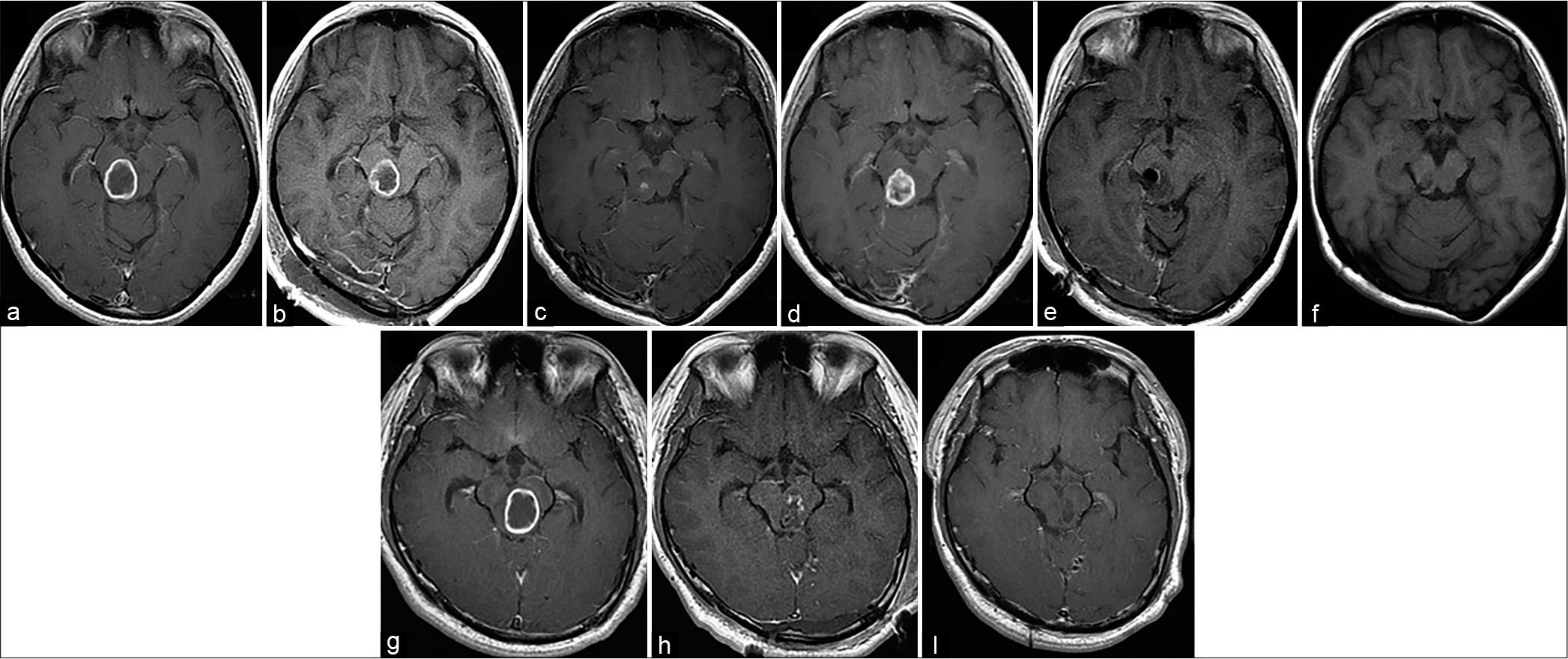
Figure 1:
Gadolinium-enhanced T1-weighted magnetic resonance imaging (GdT1WI) of Cases 1 and 2. Case 1: Preoperative GdT1WI reveals an enhanced cystic tumor on the right side of the midbrain tegmentum (a). Postoperative magnetic resonance imaging indicates partial resection of the tumor (b). GdT1WI obtained 15 months after the initial surgery reveals tumor regression (c). After 30 months, follow-up GdT1WI reveals regrowth of the enhanced tumor (d). GdT1WI obtained immediately after the second surgery indicates gross total tumor resection (e). GdT1WI obtained 3 years after the second surgery reveals no recurrence (f). Case 2: Preoperative GdT1WI reveals an enhanced cystic tumor on the left side of the midbrain tegmentum (g). Postoperative GdT1WI indicates subtotal resection of the tumor (h). GdT1WI obtained 2 years after the second surgery reveals no recurrence (i).
Case 2
An 18-year-old boy presented with a tremor in the right hand. GdT1WI MRI revealed a cystic tumor with thin-wall enhancement on the left side of the midbrain tegmentum [
Tumor specimens, DNA extraction, and pyrosequencing
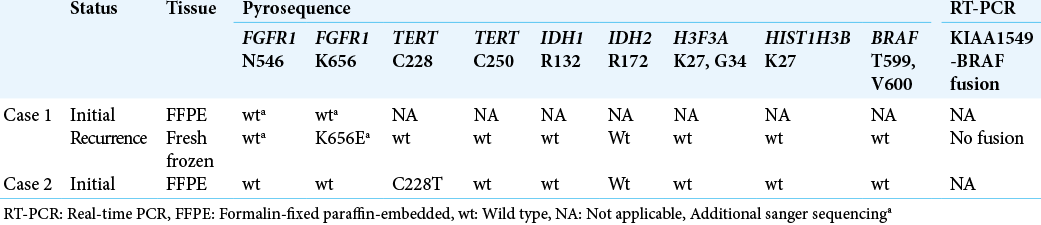
Surgical tumor specimens of the initial and recurrent tissue samples of Case 1 and the initial tissue sample of Case 2 were subjected to the central pathological review by the Japan Children’s Cancer Group. Due to the tumor location in the midbrain, the tumor specimens were small. We were only able to obtain fresh frozen tissue from the recurrent tumor in Case 1, while paraffin-embedded specimens were available for the others. DNA from the frozen tumor tissue was extracted using a DNeasy Blood and Tissue Kit (Qiagen, Tokyo, Japan), and DNA from paraffin-embedded tumor tissue was extracted using a QIAamp DNA FFPE Tissue Kit (Qiagen, Tokyo, Japan). Hotspot mutations, including IDH1 R132, IDH2 R172, BRAF T599, BRAF V600, H3F3A K27, H3F3A G34, HIST1H3B K27, FGFR1 N546, FGFR1 K656, and C228T or C250T mutations in the promoter region of TERT (TERTp), were assessed through pyrosequencing[
Histological findings
Cases 1 [
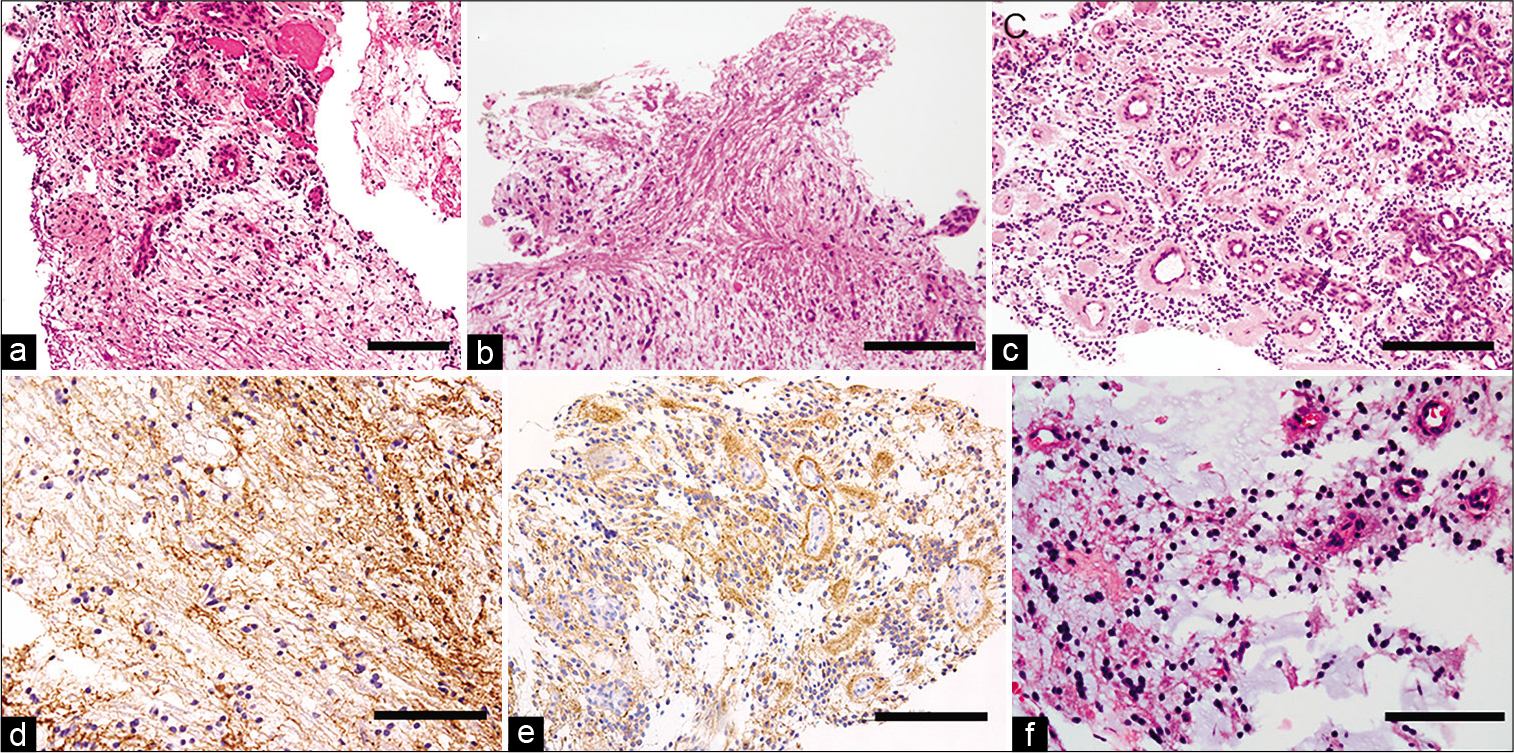
Figure 2:
Histological findings of Case 1. Hematoxylin and Eosin (HE) staining demonstrate coexistence of glial and neurocytic components (a). Higher magnification reveals the glial component (b) and neurocytic component (c). The glial component is GFAP positive (d) and the neurocytic component is synaptophysin positive (e). HE staining of the recurrent tissue does not reveal malignant changes (f). The black bar indicates 100 um.
Molecular analysis
The results of molecular analyses are summarized in [
Case 1
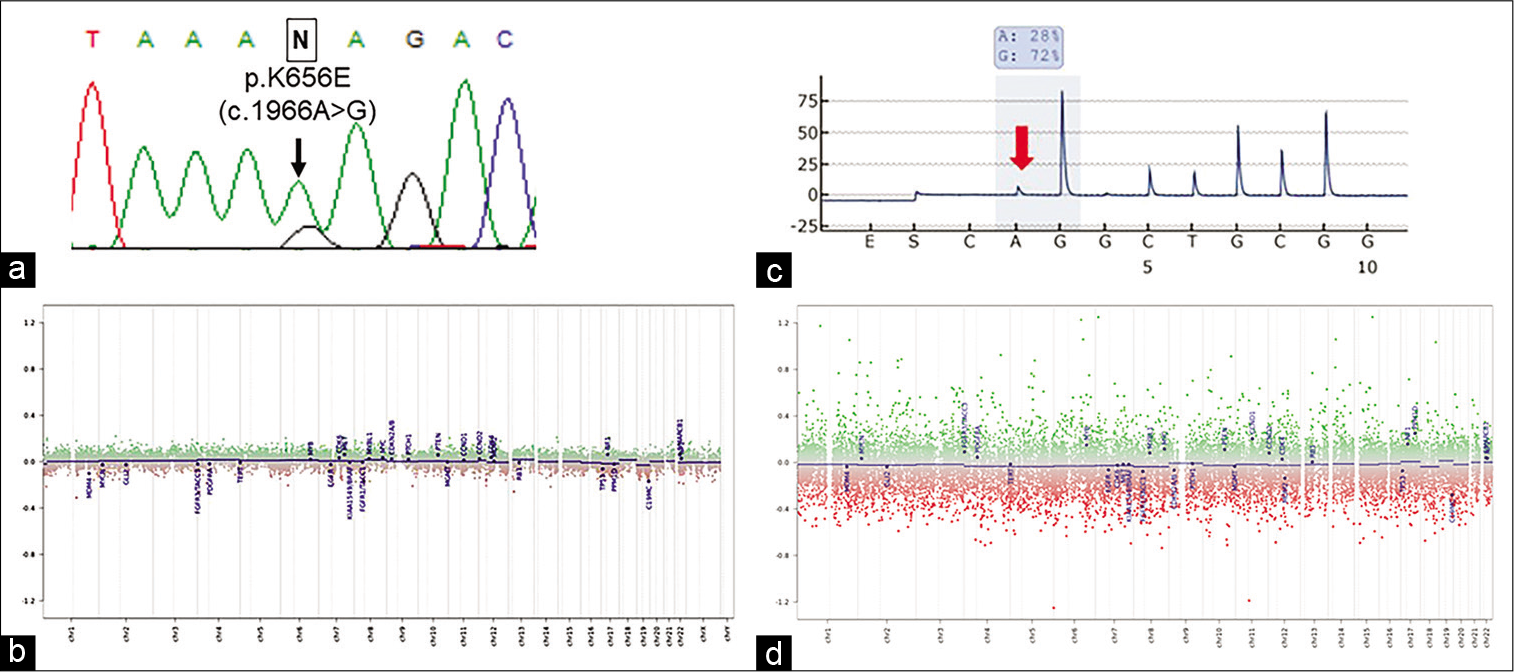
FGFR1 mutation was assessed using the initial FFPE specimen, but neither N546 nor K656 mutations were detected [
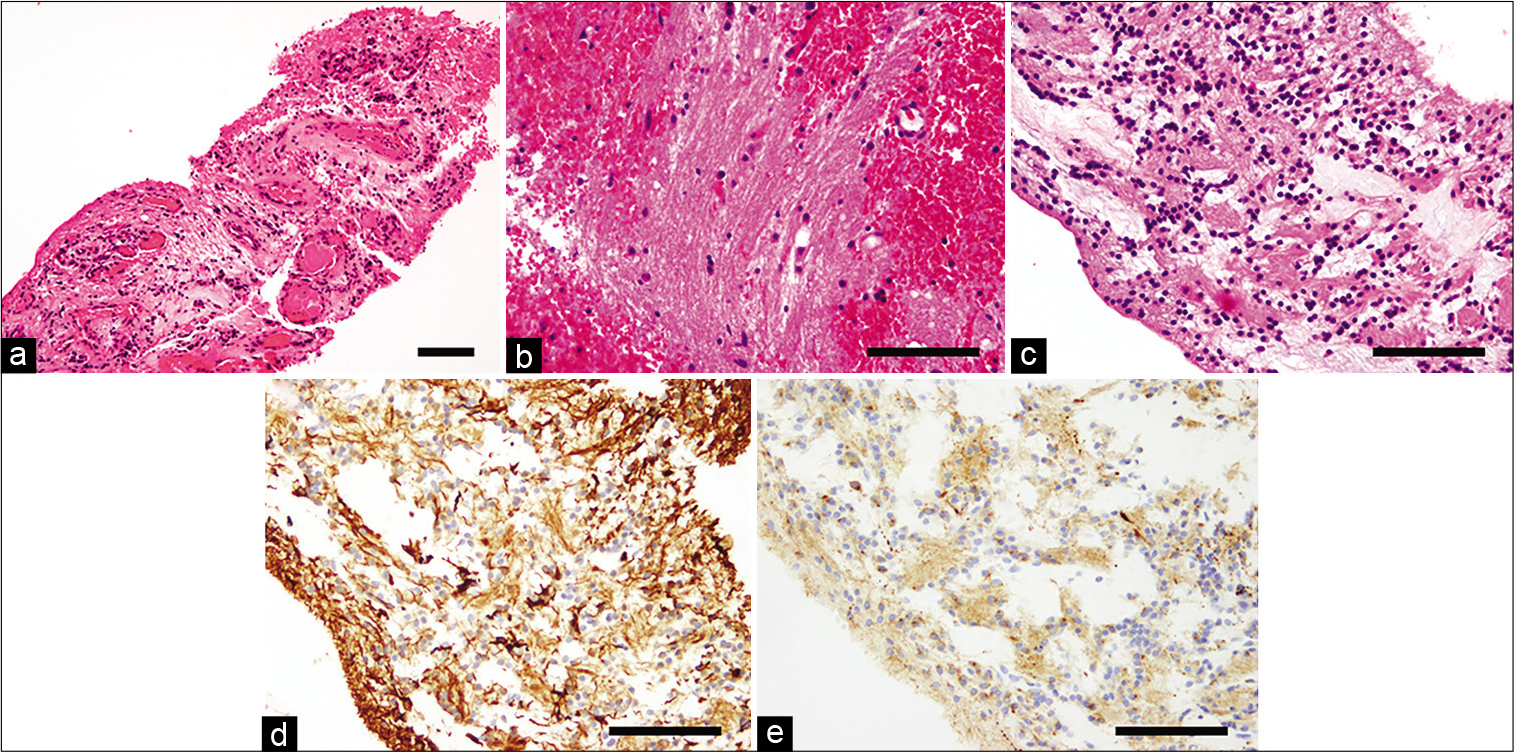
Figure 4:
Molecular analysis of Case 1 (a and b) and Case 2 (c and d). Sanger sequencing reveals FGFR1 K656E mutation (c.1966A>G, arrow) in the recurrent specimen (a), and the DNA methylation-based classification reveals flattened copy-number alterations corresponding to those of RGNT (b). Pyrosequencing reveals a mutation at the C228T of TERT promoter (c, red arrow) and the DNA methylation-based classification reveals flattened copy-number alterations corresponding to those of RGNT (d).
Case 2
Molecular analyses using the initial FFPE specimen revealed no FGFR1 mutations but revealed TERTp C228T mutation [
DISCUSSION
We have reported two cases of histologically confirmed RGNT located in the midbrain tegmentum using molecular analyses. Brainstem RGNT has a prevalence of 2.7%,[
Diffuse midline gliomas or tectal gliomas can be differential diagnoses due to the midline, brainstem, or midbrain locations. A report of adult brainstem gliomas included nine lesions located in the midbrain tectum and two in the midbrain tegmentum. One case each exhibited H3K27M positivity on immunohistochemistry,[
The initial tumors in Cases 1 and 2 did not harbor FGFR1 mutations, in contrast to the findings of Sievers et al., all of whose RGNT cases carried FGFR1 hotspot mutations.[
We found a TERTp mutation in the RGNT cases. Duan et al. analyzed TERTp mutations in spinal RGNTs found no mutations.[
CONCLUSION
We have reported two cases of cystic tumor in the midbrain tegmentum, histologically diagnosed as RGNTs. Molecular analyses showed that both cases were negative for FGFR1 mutation in the initial tumor specimens, and one case presented with TERTp mutation. The present cases indicate that RGNT in uncommon tumor locations, including the midbrain tegmentum, may molecularly differ from RGNTs in other common tumor locations, and demonstrate the importance of molecular analyses for understanding the pathophysiology of RGNTs.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
This work was in part supported by JSPS KAKENHI Grant Number 20K16873 (YN).
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
The authors would like to thank central pathological and molecular review by Japan Children’s Cancer Group, and Enago (“ http://www.enago.jp ” www.enago.jp) for the English language review. We thank M. Kitahara and Y. Hibiya for their technical support.
References
1. Allinson KS, O’Donovan DG, Jena R, Cross JJ, Santarius TS. Rosette-forming glioneuronal tumor with dissemination throughout the ventricular system: A case report. Clin Neuropathol. 2015. 34: 64-9
2. Anan M, Inoue R, Ishii K, Abe T, Fujiki M, Kobayashi H. A rosette-forming glioneuronal tumor of the spinal cord: The first case of a rosette-forming glioneuronal tumor originating from the spinal cord. Hum Pathol. 2009. 40: 898-901
3. Aoki K, Nakamura H, Suzuki H, Matsuo K, Kataoka K, Shimamura T. Prognostic relevance of genetic alterations in diffuse lower-grade gliomas. Neuro Oncol. 2018. 20: 66-77
4. Appay R, Bielle F, Sievers P, Barets D, Fina F, Boutonnat J.editors. Rosette-forming glioneuronal tumours are midline, FGFR1-mutated tumours. Neuropathol Appl Neurobiol. 2022. p. e12813
5. Arita H, Narita Y, Fukushima S, Tateishi K, Matsushita Y, Yoshida A. Upregulating mutations in the TERT promoter commonly occur in adult malignant gliomas and are strongly associated with total 1p19q loss. Acta Neuropathol. 2013. 126: 267-76
6. Arita H, Yamasaki K, Matsushita Y, Nakamura T, Shimokawa A, Takami H. A combination of TERT promoter mutation and MGMT methylation status predicts clinically relevant subgroups of newly diagnosed glioblastomas. Acta Neuropathol Commun. 2016. 4: 79
7. Bidinotto LT, Scapulatempo-Neto C, Mackay A, de Almeida GC, Scheithauer BW, Berardinelli GN. Molecular profiling of a rare rosette-forming glioneuronal tumor arising in the spinal cord. PLoS One. 2015. 10: e0137690
8. Capper D, Jones DTW, Sill M, Hovestadt V, Schrimpf D, Sturm D. DNA methylation-based classification of central nervous system tumours. Nature. 2018. 555: 469-74
9. Chakraborti S, Mahadevan A, Govindan A, Bhateja A, Dwarakanath S, Aravinda HR. Rosette-forming glioneuronal tumor--evidence of stem cell origin with biphenotypic differentiation. Virchows Arch. 2012. 461: 581-8
10. Daoud EV, Rajaram V, Cai C, Oberle RJ, Martin GR, Raisanen JM. Adult brainstem gliomas with H3K27M mutation: Radiology, pathology, and prognosis. J Neuropathol Exp Neurol. 2018. 77: 302-11
11. Duan L, Zhang Y, Fu W, Geng S. Rosette-forming glioneuronal tumor originating from the spinal cord: Report of 2 cases and literature review. World Neurosurg. 2017. 98: 875.e1-7
12. Engelhardt S, Behling F, Beschorner R, Eckert F, Kohlhof P, Tatagiba M. Frequent FGFR1 hotspot alterations in driver-unknown low-grade glioma and mixed neuronal-glial tumors. J Cancer Res Clin Oncol. 2022. 148: 857-66
13. Gessi M, Moneim YA, Hammes J, Goschzik T, Scholz M, Denkhaus D. FGFR1 mutations in Rosette-forming glioneuronal tumors of the fourth ventricle. J Neuropathol Exp Neurol. 2014. 73: 580-4
14. Ghosal N, Furtado SV, Hegde AS. Rosette forming glioneuronal tumor pineal gland and tectum: An intraoperative diagnosis on smear preparation. Diagn Cytopathol. 2010. 38: 590-3
15. Hamauchi S, Tanino M, Hida K, Sasamori T, Yano S, Tanaka S. Spinal rosette-forming glioneuronal tumor: A case report. Medicine (Baltimore). 2019. 98: e18271
16. Haryu S, Saito R, Kanamori M, Sonoda Y, Kumabe T, Watanabe M. Rosette-forming glioneuronal tumor: Rare case presented with spontaneous disappearance of contrast enhancement. NMC Case Rep J. 2015. 2: 65-7
17. Hsu C, Kwan G, Lau Q, Bhuta S. Rosette-forming glioneuronal tumour: Imaging features, histopathological correlation and a comprehensive review of literature. Br J Neurosurg. 2012. 26: 668-73
18. Jayapalan RR, Mun KS, Wong KT, Sia SF. Malignant transformation of a rosette-forming glioneuronal tumor with IDH1 mutation: A case report and literature review. World Neurosurg X. 2019. 2: 100006
19. Kikuchi Z, Shibahara I, Yamaki T, Yoshioka E, Shofuda T, Ohe R. TERT promoter mutation associated with multifocal phenotype and poor prognosis in patients with IDH wild-type glioblastoma. Neurooncol Adv. 2020. 2: vdaa114
20. Kitamura Y, Komori T, Shibuya M, Ohara K, Saito Y, Hayashi S. Comprehensive genetic characterization of rosette-forming glioneuronal tumors: Independent component analysis by tissue microdissection. Brain Pathol. 2018. 28: 87-93
21. Komori T, Scheithauer BW, Hirose T. A rosette-forming glioneuronal tumor of the fourth ventricle: Infratentorial form of dysembryoplastic neuroepithelial tumor?. Am J Surg Pathol. 2002. 26: 582-91
22. Lin CC, Mansukhani MM, Bruce JN, Canoll P, Zanazzi G. Rosette-forming glioneuronal tumor in the pineal region: A series of 6 cases and literature review. J Neuropathol Exp Neurol. 2021. 80: 933-43
23. Liu AP, Harreld JH, Jacola LM, Gero M, Acharya S, Ghazwani Y. Tectal glioma as a distinct diagnostic entity: A comprehensive clinical, imaging, histologic and molecular analysis. Acta Neuropathol Commun. 2018. 6: 101
24. Louis DN, Giannini C, Capper D, Paulus W, Figarella-Branger D, Lopes MB. cIMPACT-NOW update 2: Diagnostic clarifications for diffuse midline glioma, H3 K27M-mutant and diffuse astrocytoma/anaplastic astrocytoma, IDH-mutant. Acta Neuropathol. 2018. 135: 639-42
25. Lucas CG, Gupta R, Doo P, Lee JC, Cadwell CR, Ramani B. Comprehensive analysis of diverse low-grade neuroepithelial tumors with FGFR1 alterations reveals a distinct molecular signature of rosette-forming glioneuronal tumor. Acta Neuropathol Commun. 2020. 8: 151
26. Matsumura N, Wang Y, Nakazato Y. Coexpression of glial and neuronal markers in the neurocytic rosettes of rosette-forming glioneuronal tumors. Brain Tumor Pathol. 2014. 31: 17-22
27. Shibayama C, Doai M, Matoba M, Morikawa M, Sato H, Okada N. Spinal rosette-forming glioneuronal tumor: First case in a young child. Radiol Case Rep. 2021. 16: 3982-6
28. Sieg EP, Payne R, Langan S, Specht CS. Case report: A rosette-forming glioneuronal tumor in the tectal plate in a patient with neurofibromatosis Type I. Cureus. 2016. 8: e857
29. Sievers P, Appay R, Schrimpf D, Stichel D, Reuss DE, Wefers AK. Rosette-forming glioneuronal tumors share a distinct DNA methylation profile and mutations in FGFR1, with recurrent co-mutation of PIK3CA and NF1. Acta Neuropathol. 2019. 138: 497-504
30. Solis OE, Mehta RI, Lai A, Mehta RI, Farchoukh LO, Green RM. Rosette-forming glioneuronal tumor: a pineal region case with IDH1 and IDH2 mutation analyses and literature review of 43 cases. J Neurooncol. 2011. 102: 477-84
31. Yang C, Fang J, Li G, Li S, Ha T, Wang J. Histopathological, molecular, clinical and radiological characterization of rosette-forming glioneuronal tumor in the central nervous system. Oncotarget. 2017. 8: 109175-90