- Department of Pathology, Division of Neuropathology, University of Rochester School of Medicine, Rochester, New York, USA
- Department of Neurosurgery, University of Rochester School of Medicine, Rochester, New York, USA
Correspondence Address:
Mahlon D. Johnson
Department of Neurosurgery, University of Rochester School of Medicine, Rochester, New York, USA
DOI:10.4103/sni.sni_367_16
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Mahlon D. Johnson, Mary O’Connell, Kevin Walter, Howard Silberstein. mTOR activation is increased in pilocytic astrocytomas from older adults compared with children. 26-May-2017;8:85
How to cite this URL: Mahlon D. Johnson, Mary O’Connell, Kevin Walter, Howard Silberstein. mTOR activation is increased in pilocytic astrocytomas from older adults compared with children. 26-May-2017;8:85. Available from: http://surgicalneurologyint.com/surgicalint-articles/mtor-activation-is-increased-in-pilocytic-astrocytomas-from-older-adults-compared-with-children/
Abstract
Background:Recent studies suggest that the behavior and biology of WHO grade I pilocytic astrocytomas (PAs) in adults is different than that associated with grade I PAs in children.
Methods:We evaluated Ki-67 labeling, BRAF abnormalities, isocitrate dehydrogenase R132 immunoreactivity phosphorylation (activation) of p44/42 mitogen activated protein kinase (MAPK), and mammalian target of rapamycin (mTOR) in formalin-fixed tissue from 21 adult (18 years or older, mean age 37 years) and 10 children (mean age 9.4 years) WHO grade I PAs.
Results:The mean Ki-67 labeling was 4.8% in adults and 3.8% in children. There was no significant difference between Ki-67 labeling in children and adults or either subgroups of adults. No differences were found in phospho p44/42MAPK in adult subgroups (18–33 years and 34 and older) compared to children. Activation/phosphorylation of mTOR was biphasic in adults being significantly lower than children in young adults but significantly higher than children in older adults (age 34 and older).
Conclusions:Identifying mTOR phosphorylation/activation may represent a difference in biology and a new marker to guide chemotherapy with recently approved mTOR inhibitors.
Keywords: mTOR, pilocytic astrocytoma, p44/42MAPK
INTRODUCTION
A recent study suggests that the recurrence rate for pilocytic astrocytomas (PA) in adults may be significantly higher than that in children. Progression after gross total resection was found to be approximately 40% in adults compared to relatively infrequent in children.[
Activation of the p44/42 mitogen activated protein kinase (MAPK) has been implicated in several neoplasias[
MATERIALS AND METHODS
Pilocytic astrocytoma tissue
Thirty-one formalin-fixed paraffin embedded World Health Organization (WHO) grade I PAs from 21 adults (mean age 37 years, adults range 18–70, 10 females and 11 males) and 10 children, mean age 9.4 years (9 females and 1 male), were identified in the University of Rochester Medical Center archives and consultations with Institutional Review Board approval from 2008 to 2015 and reviewed following WHO criteria and recent findings.[
Immunohistochemistry for phospho-p44/42MAPK and phospho- mTOR in pilocytic astrocytomas
Each case was analyzed with a polyclonal antibody to phospho p44/42 MAPK (Thr 202/Tyr 204, 1:400) and human phospho-mTOR (Ser 2448, 1:100 Cell Signaling, Beverly MA.) and MAC4 universal HRP-polymer (Biocare) with diaminobenzidene (DAB) chromagen and hematoxylin counterstain (Biocare), as described previously.[
RESULTS
Comparison of pathological features of pilocytic astrocytomas in adults and children
The mean Ki-67 labeling index was 4.8% in adults and 3.8% in children. The Ki-67 labeling was not significantly different in either the 18–33 or 34 and older adults compared to children, and was not statistically different between the subgroups in adults. Neither adults nor children showed IDHR132 immunoreactivity. None of the 6 adults analyzed had a BRAFv600E mutation but one showed the BRAF-KIAA fusion. Two of the 4 tumors in children had BRAF mutations [
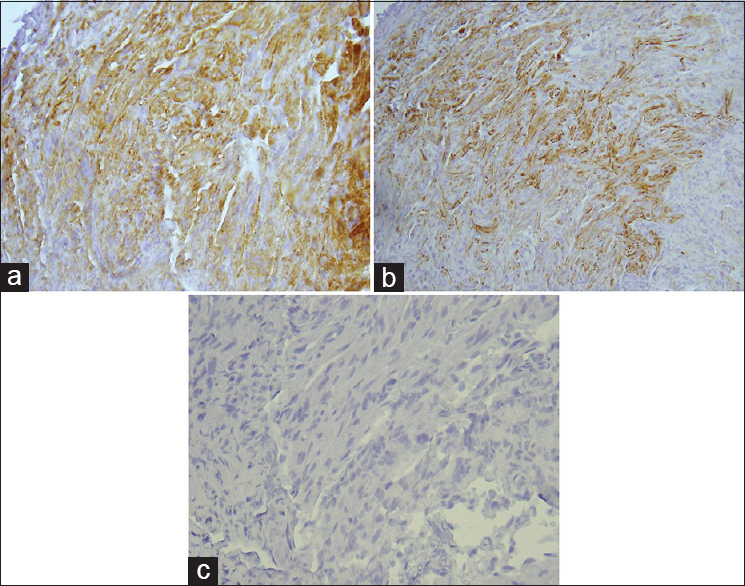
Phospho-p44/42MAPK and phospho-mTOR immunoreactivity in pilocytic astrocytomas
As summarized in
Phospho-mTOR immunoreactivity had a mean of 1.37 ± 1.18 SD in children (< 18 years of age, 0.33 ± 0.487 in young adults (18–33) and 1.36 ± 1.28 in adults 34 or greater years of age. Phospho-mTOR was biphasic in adults being significantly lower than children in young adults (P = 0.05) but significantly higher than children in older adults (age 34–70 years) (P = 0.05) [
DISCUSSION
The BRAF v600E mutation was found in only 1 of the 6 cases analyzed, which is consistent with previous reports that BRAF v600E mutations are rare in adults.[
Two studies suggest that the MEK-1-p44/42 MAPK pathway is involved in the pathogenesis of childhood PAs.[
The PI3K- PKB/Akt-mTOR pathway influences several functions central to neoplasia including cell proliferation and metabolism.[
The PI3K- PKB/Akt-mTOR pathway is also activated in many WHO grade I adult PAs, and is significantly higher in older adults compared to children. This raises the possibility that activation of this pathway may participate in the pathogenesis of PAs in the older population. Activation of mTOR is also increased in anaplastic PAs.[
Phosphorylation of p44/42MAPK and mTOR have been found to be less stable with cold ischemia after surgical removal than some other phosphoproteins.[
In summary, phosphorylation/activation of mTOR appears increased, particularly in PAs in adults 34 years of age and older. Identifying mTOR phosphorylation/activation likely represents a new marker to guide chemotherapy because several rapamycin analogues that inhibit mTOR activation are now in clinical trials or FDA approved.[
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Benjamin D, Colombi M, Moroni C, Hall MN. Rapamycin passes the torch: A new generation of mTOR inhibitors. Nature. 2011. 10: 868-80
2. Brokinkel B, Peetz-Dienhart S, Ligges S, Brentrup A, Stummer W, Paulus W. A comparative analysis of MAPK pathway hallmark alterations in pilocytic astrocytomas: Age related and mutually exclusive. Neuropathol Appl Neurobiol. 2015. 41: 258-61
3. Collins VP, Tihan T, Tihan T, Vandenberg SR, Burger PC, Hawkins C, Louis DN, Ohgaki H, Wiestler OD, Cavenee WK.editors. Pilocytic astrocytoma. Tumours of the Nervous System. Geneva, Switzerland: WHO Press; 2016. p. 80-8
4. Cykowki MD, Allen RA, Kabaly AC, Fung KM, Marshall R, Perry A. The differential diagnosis of pilocytic astrocytoma with atypical features and malignant glioma: An analysis of 16 cases with emphasis on distinguishing molecular features. J Neurooncol. 2013. 115: 477-86
5. Enralp Y, Derin D, Ozluk Y, Yavuz E, Guney E, Saip P. MAPK overexpression is associated with anthracycline resistance and increased risk for recurrence in patients with triple-negative breast cancer. Ann Oncol. 2008. 19: 669-74
6. Hasselblatt M, Riesmeier B, Lechtape B, Brentrup A, Stummer W, Albert FK. BRAF-KIAA 1549 fusion transcripts are less frequent in pilocytic astrocytomas diagnosed in adults. Neuropathol Appl Neurbiol. 2011. 37: 803-6
7. Gutmann DH, Chen YH. The molecular and cell biology of pediatric low grade gliomas. Oncogene. 2014. 33: 2019-26
8. Jacob K, Quang-Khuong DA, Jones DT, Witt H, Lambert S, Albrecht S. Genetic alterations leading to MAPK pathway activation mediate oncogene-induced senescence in sporadic pilocytic astrocytomas. Clin Cancer Res. 2011. 17: 4650-60
9. Johnson MD, O’Connell M, Vito F, Bakos RS. Increased STAT-3 and synchronous activation of Raf-1-MEK-1-MAPK, and phosphatidylinositol 3-Kinase-Akt-mTOR pathways in atypical and anaplastic meningiomas. J Neuro-Oncol. 2009. 92: 129-35
10. Jones DTW, Gromuch J, Lichter P, Witt O, Pfister SM. MAPK pathway activation in pilocytic astrocytoma. Cell Mol Life Sci. 2012. 69: 799-81
11. Jones DTW, Hutter B, Jager N, Korshunov A, Kool M, Warnatz HJ. Reccurrent somatic alterations of FGFR1 and NTRK2 in pilocytic astrocytoma. Nat Genet. 2013. 45: 927-32
12. Kieran M, Yao X, Macy M. A prospective multi-institutional Phase II study of everolimus (Rad001), an mTOR inhibitor in pediatric patients with recurrent or progressive low-grade gliomas. Ped Blood Cancer. 2013. 60: 300-10
13. McCubrey JA, Steelman LS, Chappell WH, Sun L, Davis NM, Abrams SL. Advances in targeting signal transduction pathways. Oncotarget. 2012. 3: 123456-1521
14. Nan Y, Yang S, Tian Y, Zhang W, Zhou B, Bu L, Huo S. Analysis of the expression protein profiles of lung squamous carcinoma cell using shot-gun proteomics strategy. Med Oncol. 2009. 26: 215-21
15. Nicholson KM, Anderson NG. The Akt/PKB signaling pathway in human malignancy. Cell Signal. 2002. 14: 381-95
16. Peti W, Page R. Molecular basis of MAP kinase regulation. Protein Sci. 2013. 12: 1698-710
17. Raabe EH, Lim KS, Kim JM, Meeker A, Mao XG, Nikkhah G. BRAF activation induces transformation and then senescence in human stem cells: A pilocytic astrocytoma model. Clin Cancer Res. 2011. 17: 3590-9
18. Rodriguez EF, Scheithauer BW, Giannini C, Rynearson A, Cen Ling, Hoesley B. PI3K/AKT pathway alterations are associated with clinically aggressive and histologically anaplastic subsets of pilocytic astrocytoma. Acta Neuropathol. 2011. 121: 407-20
19. Rodriguez FJ, Raabe EH. mTOR: A new therapeutic target for pediatric low-grade glioma?. CNS Oncol. 2014. 3: 89-91
20. Santarpia L, Lippman SL, El-Naggar A. Targeting the mitogen-activated protein kinase RAS-RAF signaling pathway in cancer therapy. Expert Opin Ther Targets. 2012. 16: 103-19
21. Sheppard K, Kinross KM, Soloman B, Pearson RB, Phillips WA. Targeting PI3 Kinase/AKT/mTOR signaling in cancer. Crit Rev Oncol. 2012. 17: 69-95
22. Theeler BJ, Ellezum B, Sadighi ZS, Mehta V, Tran MD, Adesina AM. Adult pilocytic astrocytomas: Clinical features and molecular analysis. Neuro-oncol. 2014. 16: 841-7
23. Vassilakopoulou M, Parisi F, Siddiqui S, England AM, Zarell ER, Anagnostou V. Preanalytic variables and phosphepitope expression in FFPE tissue: A quantitative epitope assessment after variable cold ischemic time. Lab Invest. 2015. 95: 334-41
24. Yu JSL, Cui W. Proliferation, survival and metabolism: The role of PI3K/AKT/mTOR signalling in pluripotency and cell fate determination. Development. 2015. 143: 3050-60






amh4r.com
Posted July 11, 2017, 5:32 am
Subependymal giant cell astrocytoma represen a ts another low-grade, predominantly pediatric, astrocytoma that almost always occurs in the setting of tuberous sclerosis complex, which can serve as a model for this type of targeted therapy given the recent clinical success of mTOR inhibitors.