- Department of Neurosurgery, Saint Louis University, Saint Louis, Missouri, USA
- Department of Pathology, Saint Louis University, Saint Louis, Missouri, USA
- Department of Otolaryngology, Saint Louis University, Saint Louis, Missouri, USA
Correspondence Address:
Matt Pierson
Department of Neurosurgery, Saint Louis University, Saint Louis, Missouri, USA
DOI:10.4103/2152-7806.189728
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Pierson M, Marwaha N, Guzman M, Mikulec AA, Coppens JR. Multifocal melanocytoma of the posterior fossa and subcutaneous scalp in the absence of neurocutaneous melanosis. Surg Neurol Int 01-Sep-2016;7:
How to cite this URL: Pierson M, Marwaha N, Guzman M, Mikulec AA, Coppens JR. Multifocal melanocytoma of the posterior fossa and subcutaneous scalp in the absence of neurocutaneous melanosis. Surg Neurol Int 01-Sep-2016;7:. Available from: http://surgicalneurologyint.com/surgicalint_articles/multifocal-melanocytoma-posterior-fossa-subcutaneous-scalp-absence-neurocutaneous-melanosis/
Abstract
Background:Primary leptomeningeal melanocytic neoplasms of the central nervous system are rare. Multifocal lesions typically occur in the setting of cutaneous melanosis. We present the first report of a posterior fossa melanocytoma and subcutaneous melanocytoma of intermediate grade in the absence of cutaneous melanosis.
Case Description:We present the case of a 22-year-old male with decreased hearing on the right side, ataxia, nausea, vomiting and a scalp mass. Magnetic resonance imaging (MRI) demonstrated occipital and cerebellopontine (CP) angle masses. The patient underwent gross total resection of the scalp mass and subtotal resection of the CP angle mass. Pathologic examination revealed melanocytoma with intermediate grade. The patient underwent stereotactic radiosurgery to the residual CP angle tumor. This case represents, to the author's knowledge, the first report associating a posterior fossa melanocytoma with a subcutaneous melanocytoma of intermediate grade in the absence of cutaneous melanosis.
Conclusion:This case introduces the first report of a new variant of multifocal melanocytoma which is not confined to the central nervous system.
Keywords: Multifocal melanocytoma meningeal melanosis, radiosurgery, subcutaneous melanocytoma
INTRODUCTION
Primary leptomeningeal melanocytic neoplasms of the central nervous system (CNS) include a spectrum of entities, which include diffuse lesions (leptomeningeal melanocytosis and melanomatosis), as well as circumscribed lesions (meningeal melanocytoma and primary malignant melanoma). Meningeal melanocytomas are derived from the melanocytes of the leptomeninges in the CNS, and were first described as benign focal lesions by Lima and Tio.[
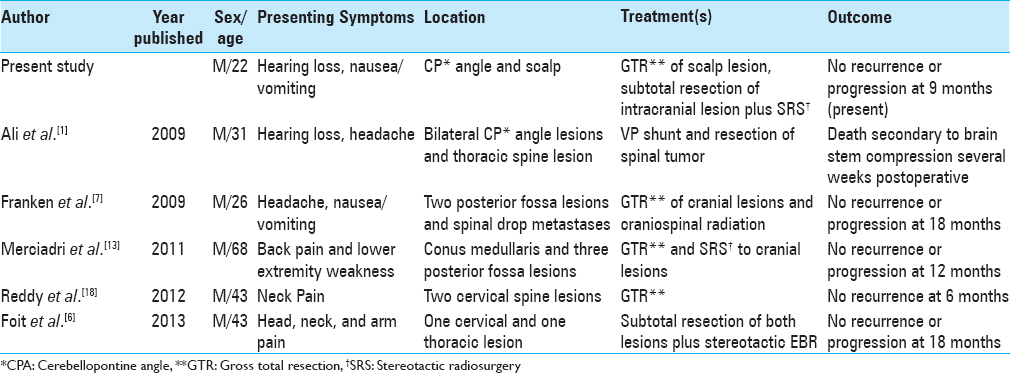
Multifocal meningeal melanocytomas at the time of diagnosis have been reported in five cases in the published literature. All described cases were contained to the CNS in the form of posterior fossa and spinal lesions. We present the case of a young male with multifocal disease in the form of a cerebellopontine intradural meningeal melanocytoma as well as a subcutaneous occipital melanocytoma. Intraoperative leptomeningeal hyperpigmentation of the surrounding dura was noted but did not meet the criteria for melanocytosis. The patient did not have cutaneous anomalies. This is the first case report of a multifocal CNS meningeal melanocytoma with a subgaleal extracranial melanocytoma described without a context of cutaneous melanosis.
CASE REPORT
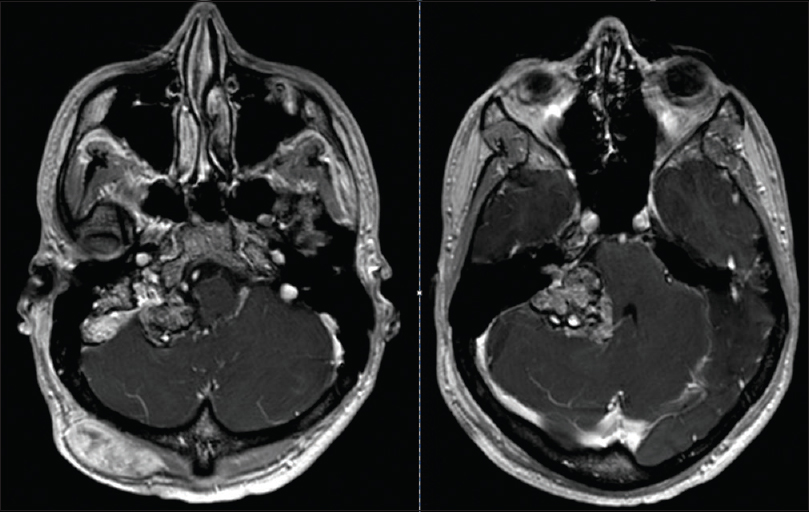
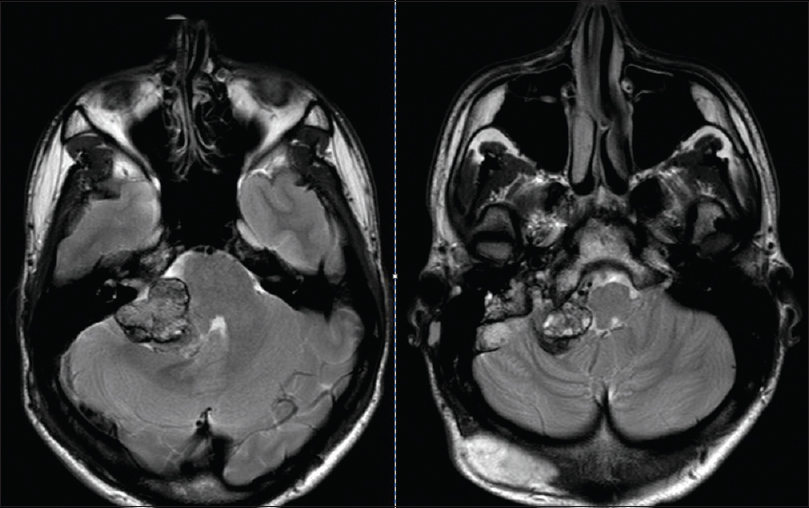
A 22-year-old male presented to our institution's emergency room with decreased hearing on the right side, ataxia, nausea, and vomiting. On physical examination, an occipital painless subcutaneous mass was noted and audiogram revealed non-serviceable hearing on the right side. A magnetic resonance image (MRI) of brain with contrast demonstrated two separate lesions with similar signal characteristics. A subcutaneous occipital mass measuring 4.8 × 2.9 × 4.1 cm was noted as well as an intradural extraxial 2.6 × 2 × 5.2 cm CP angle mass. There was extension of the CP angle mass into the enlarged jugular foramen and invasion of the internal jugular vein. Both lesions were T1 hyperintense enhancing lesions with a mixed iso and hypointense signal on T2 [Figures
Surgical resection of the two lesions was performed at our institution. The posterior fossa mass was approached through a right translabyrinthine approach and the subcutaneous lesion was resected in the same setting with a separate incision. Electrophysiologic monitoring with cranial nerve monitoring was performed. The extracranial lesion was resected first and did not show any continuity to the intracranial lesion.
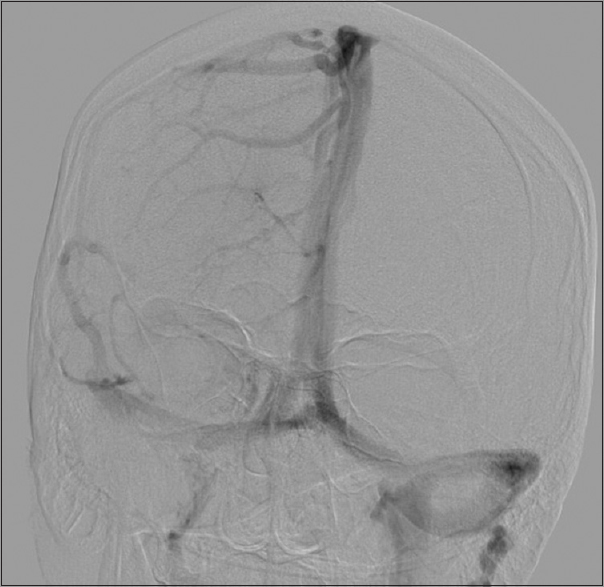
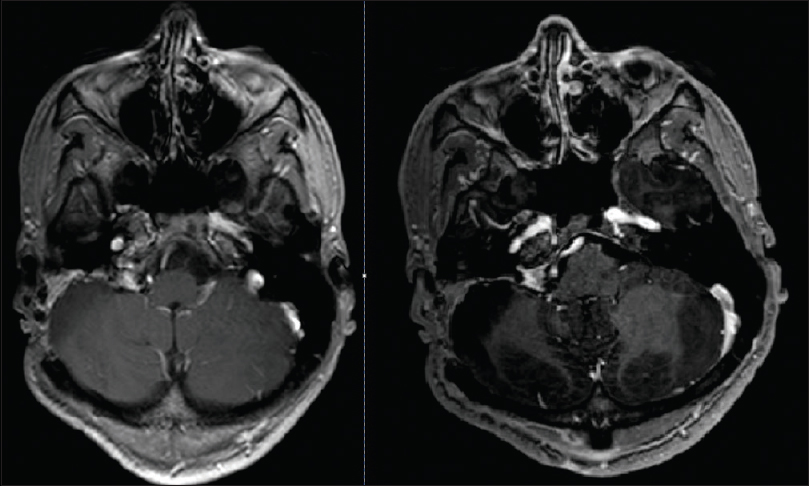
A posterior petrosal translabyrinthine approach was utilized for the intracranial lesion which permitted presigmoid access as well as exposure of the sigmoid sinus and jugular bulb. The dura showed extensive patchy areas of melanocytic coloration over the entirety of the surgical exposure. The intradural lesion was readily visible with invasion of the sigmoid sinus. The sigmoid sinus was opened and found to be occluded with tumor. The sinus was ligated on either side of the tumor invasion and sacrificed. The mass was debulked using a combination of ultrasonic aspiration and suction. The capsule was dissected-off of the lateral cerebellum, brainstem, and cranial nerve 7 despite its adherence. Dissection of the tumor to the lower cranial nerves could not be completely achieved and a subtotal resection was performed. Closure was performed with a dural onlay followed with the use of abdominal fat graft after waxing the surrounding bony structures. A lumbar drain was used postoperatively for 5 days. The patient postoperatively was noted to have nystagmus and a facial nerve function consistent with a House–Brackmann grade 2 which recovered within a month. The patient was discharged home on postoperative day 6. The patient was treated with stereotactic radiosurgery at 6 months (22 Gray in three fractions) and has been stable on MRI imaging for 12 months [
Pathology
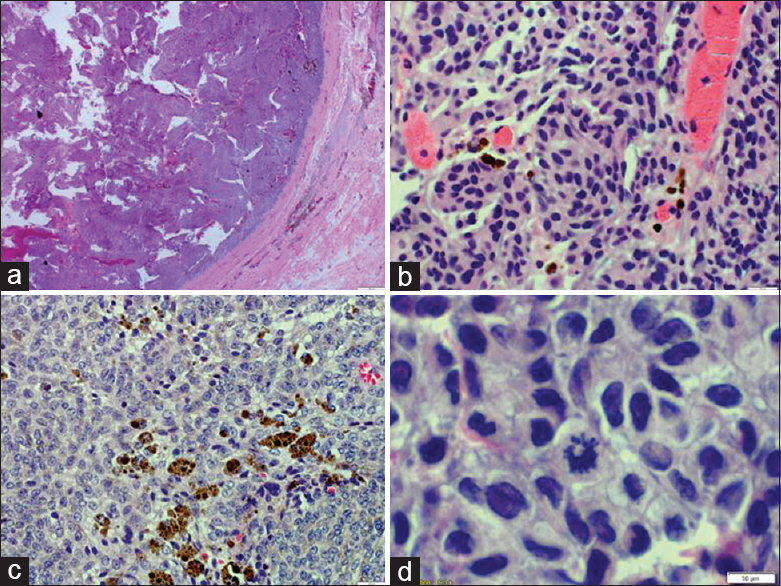
Gross and microscopic examination of both of the lesions demonstrated similar histomorphological features and immunohistochemical profile confirming the melanocytic origin. The tumor cells were arranged in a nested pattern and showed mild pleomorphism, although rare mitotic figures were noted. Marked intracellular and extracellular melanin deposition was noted [
DISCUSSION
CNS melanocytomas are tumors that arise from leptomeningeal melanocytes which are embryologically derived from neural crest cells. They are classified as benign circumscribed lesions within the spectrum of primary leptomeningeal melanocytic neoplasms. They occur across all age groups with a slight predilection for females. In the CNS, they are usually intradural extra-axial lesions that may occur intracranially as well as in the spine. The two most common intracranial locations are the posterior fossa and Meckel's cave. Spinal melanocytomas typically occur in the cervical spine followed by the thoracic spine.[
Meningeal melanocytomas are usually benign lesions that are encapsulated and do not present with direct invasion of the surrounding CNS. They are classically described by MRI imaging as hyperintense on T1-weighted images, hypointense on T2-weighted images, hyperintense on fluid-attenuated inversion recovery (FLAIR) images, and enhance homogeneously with gadolinium, as seen in this case [Figures
The benign nature of melanocytomas has been questioned based on the description of an intermediate grade. An intermediate grade is defined microscopically with the presence of mitoses (1–3 mitoses per 10 HPFs and MIB-1 LI ranging 1–4%) and/or microscopic CNS invasion.[
Treatment strategies in meningeal melanocytoma should aim for a complete resection of the lesion when feasible. If a subtotal resection is accomplished due to CNS invasion and adherence to critical structures, observation or radiosurgery should be offered. Patients should be followed closely with serial imaging of the entire CNS. It is now suggested that melanocytomas progress to melanoma within 3 months to 12 years.[
Multifocal melanocytomas of the CNS was first described as an entity in 2009 by Ali et al. when reporting a case of bilateral CP angle melanocytomas with a thoracic melanocytoma at the time of diagnosis. A total of five cases have been described in the combination of a posterior fossa mass with a spinal lesion or multiple spine lesions.[
This entity is thought to be different from cases that have demonstrated leptomeningeal spread after total or subtotal resections of a melanocytoma.[
Case reports of melanocytomas have sometimes also commented on the presence of diffuse leptomeningeal hyperpigmentation of the surrounding dura, as seen in our case.[
Melanocytomas are known to recur locally even after complete resection. Five year overall survival rates are 83% after complete resection and 40% in incomplete resection without radiation therapy.[
This is the first case report describing multifocal disease with an intradural lesion and a subcutaneous mass at the time of diagnosis. Their relationship is not understood but strongly suggests multifocal disease.
Our findings support the concept of multifocal melanocytomas, which may not be restricted within the CNS, as previously described.
CONCLUSION
We introduce a new variant of multifocal melanocytoma in a patient which was not confined to the CNS. This is the first report associating a posterior fossa melanocytoma with a subcutaneous melanocytoma of intermediate grade in the absence of cutaneous melanosis. Its clinical behavior appears to be similar to other cases of multifocal disease described in the literature.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Ali Y, Rahme R, Moussa R, Abadjian G, Menassa-Moussa L, Samaha E. Multifocal meningeal melanocytoma: A new pathological entity or the result of leptomeningeal seeding?. J Neurosurg. 2009. 111: 488-91
2. Alwatban J, Tampieri D, Salazar A, Melancon D, Duong H. MRI of leptomeningeal melanocytosis in a patient with neurofibromatosis. J Comput Assist Tomogr. 1997. 21: 38-40
3. Brat DJ, Giannini C, Scheithauer BW, Burger PC. Primary melanocytic neoplasms of the central nervous systems. Am J Surg Pathol. 1999. 23: 745-54
4. Bydon A, Gutierrez JA, Mahmood A. Meningeal melanocytoma: An aggressive course for a benign tumor. J Neurooncol. 2003. 64: 259-63
5. Fagundes-Pereyra WJ, de Sousa L, Carvalho GT, Pittella JE, de Sousa AA. Meningeal melanocytoma of the posterior fossa: Case report and literature review. Surg Neurol. 2005. 63: 269-73
6. Foit NA, Neidert MC, Woernle CM, Rushing EJ, Krayenbuhl N. Bifocal extra- and intradural melanocytoma of the spine: Case report and literature review. Eur Spine J. 2013. 22: S521-5
7. Franken SP, Setz-Pels W, Smink-Bol M, Gijtenbeek JM, Nanda D, Van Der Maazen RW. Unusual case of bifocal leptomeningeal melanocytoma in the posterior fossa with seeding in the spinal canal. Br J Radiol. 2009. 82: e182-8
8. Koch HJ, Roeber S, Zimmermann UW, Schafer C, Villarrubia V, Kuchelmeister K. [Spinal and cerebral leptomeningeal seeding from a melanocytoma of the cerebello-pontine angle]. Wien Med Wochenschr. 2005. 155: 360-4
9. Koenigsmann M, Jautzke G, Unger M, Theallier-Janko A, Wiegel T, Stoltenburg-Didinger G. June 2002: 57-year-old male with leptomeningeal and liver tumors. Brain Pathol. 2002. 12: 519-21
10. Kurita H, Segawa H, Shin M, Ueki K, Ichi S, Sasaki T. Radiosurgery of meningeal melanocytoma. J Neurooncol. 2000. 46: 57-61
11. Limas C, Tio FO. Meningeal melanocytoma (“melanotic meningioma”).Its melanocytic origin as revealed by electron microscopy. Cancer. 1972. 30: 1286-94
12. Liubinas SV, Maartens N, Drummond KJ. Primary melanocytic neoplasms of the central nervous system. J Clin Neurosci. 2010. 17: 1227-32
13. Merciadri P, Secci F, Sbaffi PF, Zona G. Multifocal meningeal melanocytoma of the conus medullaris. Acta Neurochir. 2011. 153: 2283-5
14. Rades D, Heidenreich F, Tatagiba M, Brandis A, Karstens JH. Therapeutic options for meningeal melanocytoma. Case report. J Neurosurg. 2001. 95: S225-31
15. Rades D, Schild SE. Dose-response relationship for fractionated irradiation in the treatment of spinal meningeal melanocytomas: A review of the literature. J Neurooncol. 2006. 77: 311-4
16. Rades D, Schild SE, Tatagiba M, Molina HA, Alberti W. Therapy of meningeal melanocytomas. Cancer. 2004. 100: 2442-7
17. Rades D, Tatagiba M, Brandis A, Dubben HH, Karstens JH. [The value of radiotherapy in treatment of meningeal melanocytoma]. Strahlenther Onkol. 2002. 178: 336-42
18. Reddy R, Krishna V, Sahu BP, Uppin M, Sundaram C. Multifocal spinal meningeal melanocytoma: An illustrated case review. Turk Neurosurg. 2012. 22: 791-4
19. Roser F, Nakamura M, Brandis A, Hans V, Vorkapic P, Samii M. Transition from meningeal melanocytoma to primary cerebral melanoma. Case report. J Neurosurg. 2004. 101: 528-31
20. Shownkeen HN, Harmath C, Thomas C. Multiform cervical melanocytoma: A case report. Neuroradiology. 2002. 44: 1008-10
21. Smith AB, Rushing EJ, Smirniotopoulos JG. Pigmented lesions of the central nervous system: Radiologic-pathologic correlation. Radiographics. 2009. 29: 1503-24
22. Uozumi Y, Kawano T, Kawaguchi T, Kaneko Y, Ooasa T, Ogasawara S. Malignant transformation of meningeal melanocytoma: A case report. Brain Tumor Pathol. 2003. 20: 21-5
23. Wang F, Li X, Chen L, Pu X. Malignant transformation of spinal meningeal melanocytoma. Case report and review of the literature. J Neurosurg Spine. 2007. 6: 451-4
24. Wang H, Zhang S, Wu C, Zhang Z, Qin T. Melanocytomas of the central nervous system: A clinicopathological and molecular study. Eur J Clin Invest. 2013. 43: 809-15