- Department of Neurosurgery, Tenri Hospital, Tenri, Nara, Japan
Correspondence Address:
Satoshi Shitara
Department of Neurosurgery, Tenri Hospital, Tenri, Nara, Japan
DOI:10.4103/sni.sni_348_17
Copyright: © 2018 Surgical Neurology International This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.How to cite this article: Satoshi Shitara, Tomoo Tokime, Yoshinori Akiyama. Multinodular and vacuolating neuronal tumor: A case report and literature review. 19-Mar-2018;9:63
How to cite this URL: Satoshi Shitara, Tomoo Tokime, Yoshinori Akiyama. Multinodular and vacuolating neuronal tumor: A case report and literature review. 19-Mar-2018;9:63. Available from: http://surgicalneurologyint.com/surgicalint-articles/multinodular-and-vacuolating-neuronal-tumor-a-case-report-and-literature-review/
Abstract
Background:Multinodular and vacuolated neuronal tumor (MVNT) is a benign neuronal tumor that is newly recognized as architectural appearance that may be related to ganglion cell tumors in 2016 World Health Organization Classification of Tumors of the Central Nervous System. Herein, we report a case of MVNT in a 60-year-old man with a thorough literature review.
Case Description:A 60-year-old male was pointed out the presence of intracerebral neoplasm located in left frontal lobe by a comprehensive medical examination. We suspected dysembryoplastic neuroepithelial tumors and proposed him to wait and see, but he wished to undergo surgery for diagnosis. We performed en bloc resection and pathological findings were consistent with MVNT. He was discharged on the 8th day after the operation without any complications. He remained stable without recurrence at the 16-month postoperative follow-up.
Conclusions:Further studies may be helpful to fully understand the radiological and histological findings of MVNT development. As a result, we will be able to prevent the aggressive treatment if we established their major features.
Keywords: Brain tumor, multinodular and vacuolated pattern, radiographic characteristics
INTRODUCTION
Multinodular and vacuolated pattern is newly recognized as architectural appearance that may be related to ganglion cell tumors in 2016 World Health Organization Classification of Tumors of the Central Nervous System.[
CASE REPORT
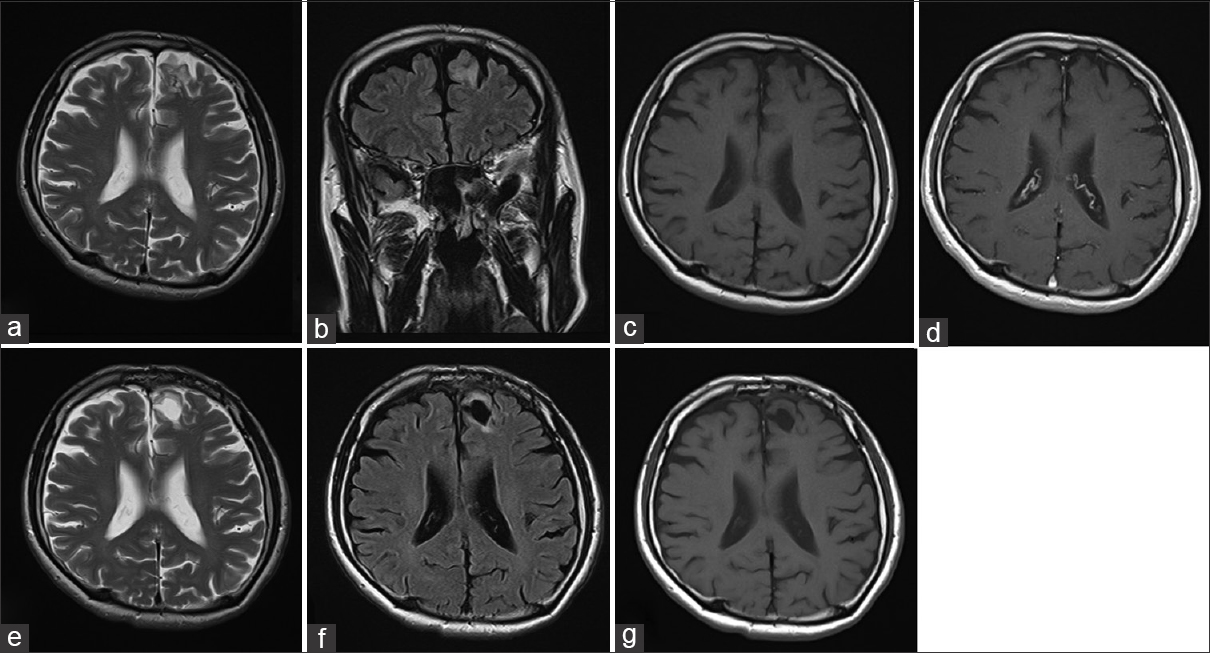
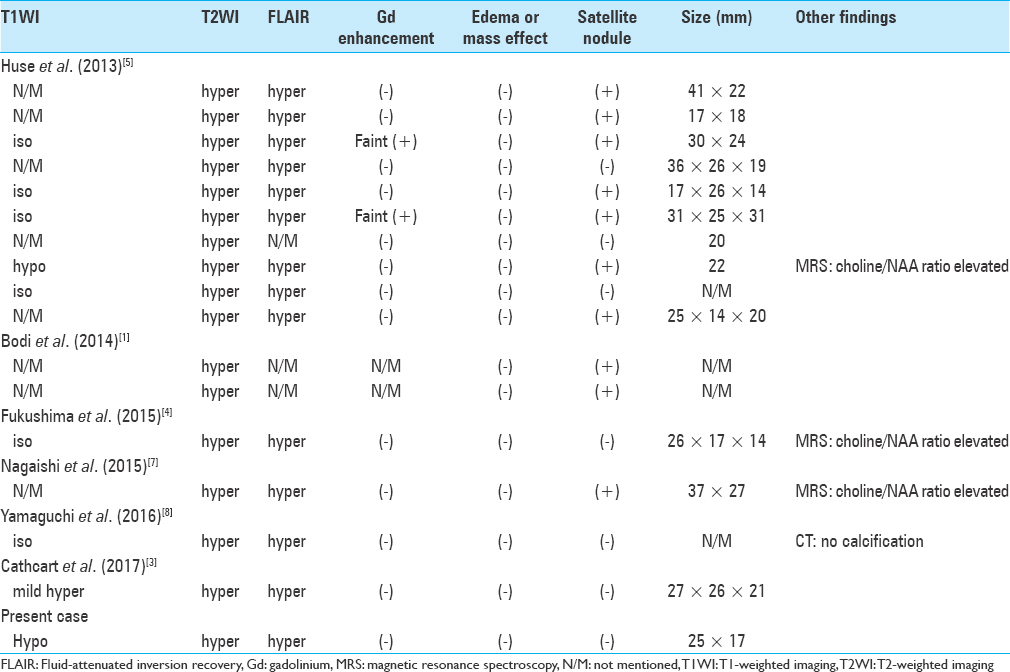
A 60-year-old Japanese man underwent a comprehensive medical examination with brain magnetic resonance imaging (MRI). The neurological examination indicated no significant findings. However, MRI revealed a 25 mm × 17 mm, nonenhanced lesion with gadolinium in the left superior frontal gyrus as a hypointense mass in T1-weighted imaging (T1WI) and hyperintense in T2-weighted imaging (T2WI) and fluid attenuated inversion recovery (FLAIR) without any mass effect or edema [Figure
Figure 1
Preoperative MRI revealed the lesion identified in the left superior frontal gyrus (a-d). The lesion showed hyperintensity on T2WI (a) and FLAIR (b). The lesion demonstrated slight hypointensity on T1WI (c) and does not exhibit mass effect, contrast enhancement (d), or associated edema. Postoperative MRI (16 months after operation) showed total resection and the removed cavity with no evidence of tumor recurrence by T2WI (e), FLAIR (f), and T1WI (g)
The tumor was exposed via a transcortical approach and we could not identify the obvious boundary between tumor and normal brain. Total en bloc resection was performed with intraoperative navigation. The postoperative course was uneventful and he was discharged on the 8th day after the operation without any complications. He remained stable without recurrence of the lesion on MRI at the 16-month postoperative follow-up [Figure
Histopathological findings
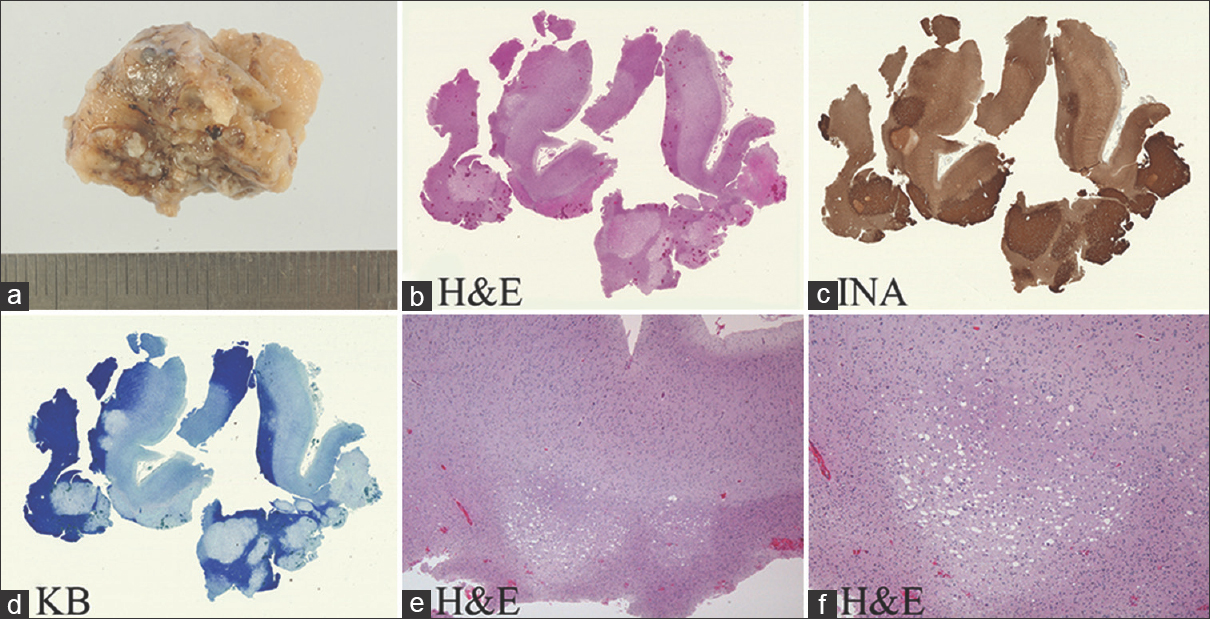
We could resect the tumor en bloc and performed total resection of tumor [
Figure 2
The lesion was resected in an en bloc fashion (a). Microscopy with low power magnification demonstrating a well-demarcated subcortical lesion abutting gray and white matter (b, hematoxylin and eosin stain (H and E)). Alpha-internexin (INA) expression is detected in tumor stroma (c) and Kluber-Barrera (KB) staining confirms the absence of myelin in the tumor lesion (d). The lesion demonstrates clear delineation from the surrounding brain without evidence of infiltration (e-f, H and E)
Figure 3
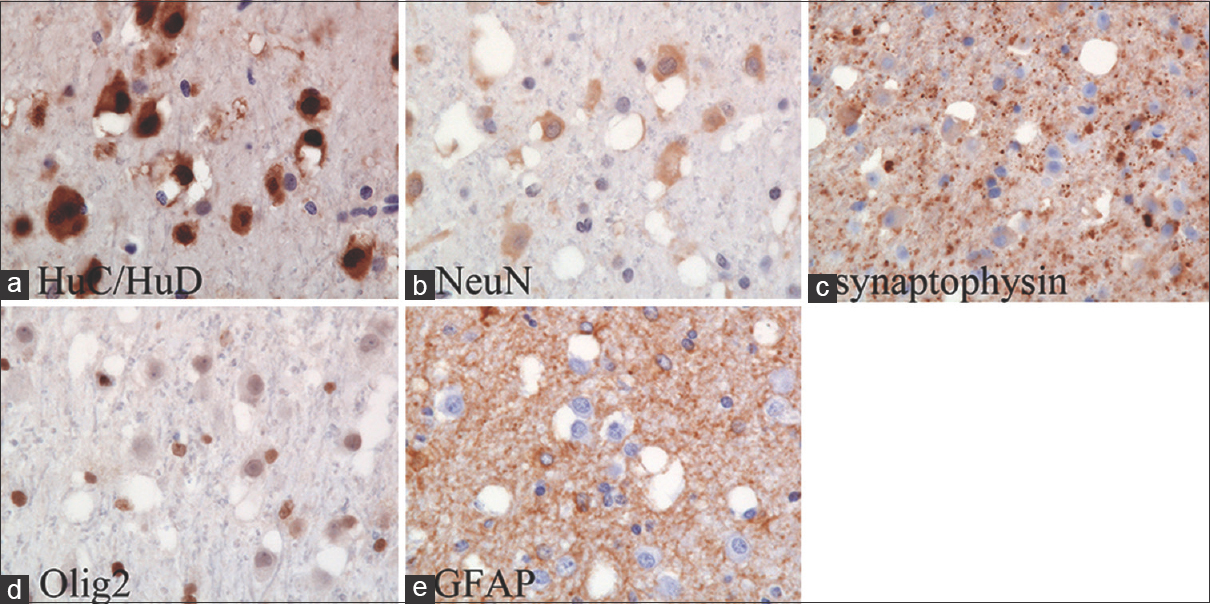
The neuronal tumor cells are intensely stained by HuC/HuD (a), but negatively or weakly stained for neuronal nuclear antigen (NeuN) (b), synaptophysin (c) and nuclear oligodendrocyte transcription factor (Olig2) (d). The ganglioid cells are unreactive for glial fibrillary acidic protein (GFAP) (e)
DISCUSSION
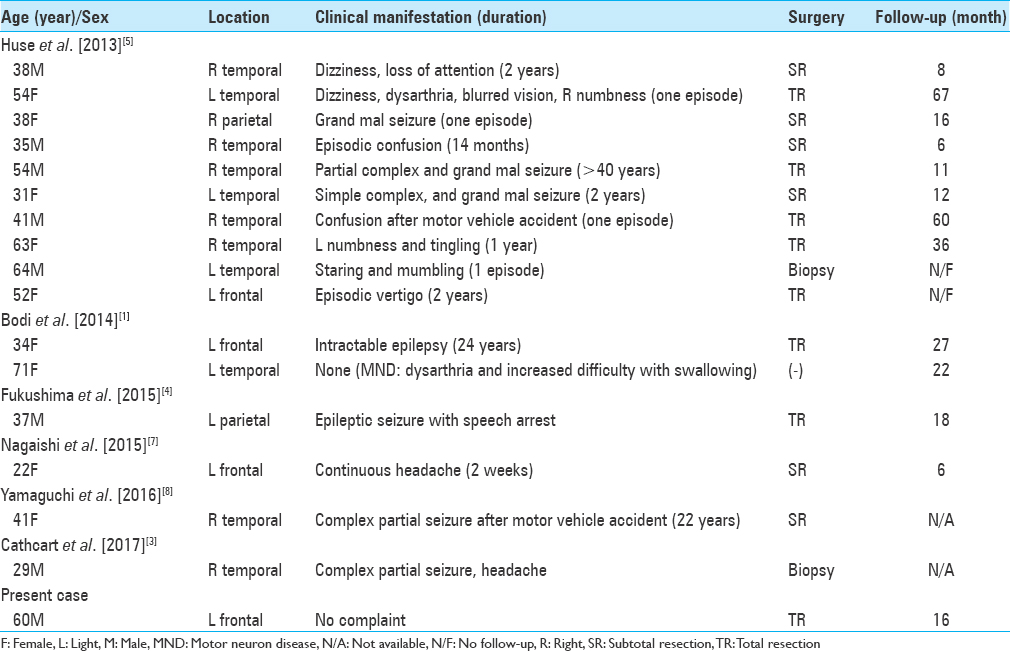
MVNTs tend to be recognized by the presence of seizure or seizure equivalents, but our case is incidentally found by a comprehensive medical examination. The lesions had potentially suspect of gliomas or the patients suffered from some neurologic complaints, then surgical extirpations were made to remove the lesions and to determine the definite diagnosis in almost all cases except one case.[
The differential diagnosis from radiological findings included dysembryoplastic neuroepithelial tumor (DNT), low-grade glioma, cortical dysplasia, hamartoma, and so on. The lesions show small bubbly appearing indolent subcortical tumor and usually have difficulty to be identified in computed tomography. On MRI, the lesions show iso- or hypointense on T1WI, hyperintense on T2WI, and increased signal in FLAIR sequences as we can identify this appearance in case of DNTs.[
As the pathological diagnosis accompanies with some difficulties as previous authors discussed, the establishment of definite imaging characterization may help to avoid invasive surgical interventions.[
CONCLUSION
Although extremely rare and usually nonfatal, MVNT should be considered in the differential diagnosis of multinodular lesion with no edema in the subcortical white matter. The clinician can suggest the best way to manage the rare entity by well-understanding of the neuro-radiologic behavior of MVNTs.
Declaration of patient consent
The patient and his family have given the necessary consent for the case report to be published.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Bodi I, Curran O, Selway R, Elwes R, Burrone J, Laxton R. Two cases of multinodular and vacuolating neuronal tumour. Acta Neuropathol Commun. 2014. 20: 2-7
2. Bulakbasi N, Kocaoglu M, Sanal TH, Tayfun C. Dysembryoplastic neuroepithelial tumors: Proton MR spectroscopy, diffusion and perfusion characteristics. Neuroradiology. 2007. 49: 805-
3. Cathcart SJ, Klug JR, Helvey JT, L White M, Gard AP, McComb RD. Multionodular and vacuolating neuronal tumor: A rare seizure-associated entity. Am J Surg Pathol. 2017. 41: 1005-10
4. Fukushima S, Yoshida A, Narita Y, Arita H, Ohno M, Miyakita Y. Multinodular and vacuolating neuronal tumor of the cerebrum. Brain Tumor Pathol. 2015. 32: 131-6
5. Huse JT, Edgar M, Halliday J, Mikolaenko I, Lavi E, Rosenblum MK. Multinodular and vacuolating neuronal tumors of the cerebrum: 10 cases of a distinctive seizure-associated lesion. Brain Pathol. 2013. 23: 515-24
6. Louis DN, Perry A, Reifenberger G, von Deimling, Figarella-Branger D, Cavenee WK. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016. 131: 803-20
7. Nagaishi M, Yokoo H, Nobusawa S, Fujii Y, Sugiura Y, Suzuki R. Localized overexpression of alpha-internexin within nodules in multinodular and vacuolating neuronal tumors. Neuropathology. 2015. 35: 561-8
8. Yamaguchi M, Komori T, Nakata Y, Yagishita A, Morino M, Isozaki E. Multinodular and vacuolating neuronal tumor affecting amygdala and hippocampus: A quasi-tumor?. Pathol Int. 2016. 66: 34-41